Outlook 2024
Annual Industry Ranking And Forecast
VIEW NOWHBW Insight is part of Pharma Intelligence UK Limited
This site is operated by Pharma Intelligence UK Limited, a company registered in England and Wales with company number 13787459 whose registered office is 5 Howick Place, London SW1P 1WG. The Pharma Intelligence group is owned by Caerus Topco S.à r.l. and all copyright resides with the group.
This copy is for your personal, non-commercial use. For high-quality copies or electronic reprints for distribution to colleagues or customers, please call +44 (0) 20 3377 3183
Printed By

Views from industry leaders and experts on the biggest trends, challenges and opportunities facing consumer health in the new year.

A National Advertising Division review of claims by LVMH-owned Fenty Skin determined makeup- and dirt-removal representations were substantiated by a study and subject questionnaire, but demo videos from paid endorsers must include disclosures about material connections in accordance with FTC guidelines.

Decisions by “big three” PBMs suggest they’re taking proactive steps to comply with potential HRSA rulemaking to require most health plans, under Affordable Care Act regulations, to cover Opill and other types of OTC birth control without cost sharing.

HiSmile appeals NAD recommendation following review on P&G challenge to discontinue ad claims on its website and in social media videos stating peroxide-containing whitening products are “painful” or cause pain, break down and impact gums and teeth, or “damage” gums.

The Independent Beauty Association does not yet have insight into what ingredients will be listed in the US FDA’s proposed rulemaking for cosmetic allergen labeling – due by 29 June – but is optimistic the agency will consult industry when it comes to setting the compliance timeline. Meanwhile, industry stakeholders are pushing for international harmonization in the agency’s cosmetic GMPs rulemaking.

Without FDA regulatory pathway for hemp’s lawful use in supplements and food, some states are imposing more-stringent regulations while others allow sales of intoxicating ingredients not considered controlled substances as hemp derivatives.
US Cannabis Council says farm bill reauthorization is “key opportunity to tackle the national crisis caused by unregulated intoxicating hemp products” by limiting the variety of hemp derivatives which qualify as de-scheduled.

Personal care and cosmetic product trademark filings compiled from the Official Gazette of the US Patent and Trademark Office, Class 3.

Artificial intelligence opportunities are on the rise for marketers, including in the beauty and personal-care sectors. Reed Smith partner Stacy Marcus offers advice on staying compliant with US consumer protection and copyright laws in this rapidly changing world.
There’s no simple answer to the question of how to make medicines packaging sustainable, says international recycling consultancy and Veolia Group subsidiary Circpack. “Manufacturers need to assess what works best for them, and recyclers will do the same,” Circpack analyst and circular packaging expert Filipe Vieira de Castro tells HBW Insight in this exclusive Q&A. “The best thing we can all do is put effort into finding common ground between systems, which comes down to better understanding the complexities between them.”
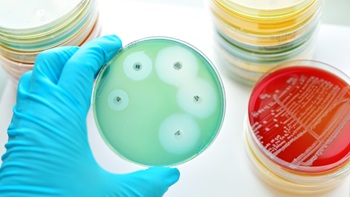
The EU Parliament's Environment, Public Health and Food Safety committee proposes a “compromise amendment” to the new pharmaceutical directive that would include only antimicrobials for which there is an “identified risk of antimicrobial resistance” to the prescription requirement.
The EU Parliament's Environment, Public Health and Food Safety committee takes a compromise position with regards to the Packaging and Packaging Waste Directive. Medicines and medical devices should be exempt, but only until 2035, at which point the European Commission should check whether the development of materials and the recycling process have progressed, and may adjust this exemption accordingly.

Haleon has rolled out 1.2bn recycle-ready tubes across the world, an achievement that the firm said is a “milestone” on its “journey towards realizing a circular economy for its product packaging.” Haleon is also introducing a more sustainable manufacturing process for several variants of its Sensodyne toothpaste brand, starting in the UK.

Artificial intelligence opportunities are on the rise for marketers, including in the beauty and personal-care sectors. Reed Smith partner Stacy Marcus offers advice on staying compliant with US consumer protection and copyright laws in this rapidly changing world.

“Companies can innovate right now, and they have confidence in these tools, these [non-animal] approaches, and they feel they can put these products on the market safely,” said Erin Hill, CEO and president of the International Collaboration on Cosmetics Safety. “The holdup is that they can’t register these new products, because there’s a lack of confidence in the regulatory community.”

Using AI-based programs to collect and store consumer information risks running afoul of new health privacy laws cropping up in US states. Lack of federal regulation or guidance on the issue is one of the biggest challenges for beauty firms deploying AI, according to Stacy Marcus, partner at Reed Smith LLP.

In this episode, HBW Insight’s Europe and US editors bring their expertise to bear on the current the trend towards standalone OTC companies in global consumer health. We look at four major players: Haleon, which separated from GSK almost two years ago; Kenvue, soon to celebrate its first anniversary as a new company; Sanofi Consumer Healthcare, which is poised to split from its pharma parent; and Bayer, which has decided to buck the trend, holding on to its consumer health division. We discuss some of the advantages of becoming a standalone company, for example in leaning into a wider concept of self-care.
A “great consumer response” to TheraTears in the UK leads Prestige Consumer Healthcare to add TheraTears 5-in-1 Irritation and Redness eye drops to the range.
Nutritional product business had 5.1% Q1 sales growth and is like Abbott’s other segments, “super well-aligned to the global demographics and trends in health care,” says CEO Ford. But as it defends complaints of damages from powder formulas made at facility found with unsafe levels of bacterial contaminants, Abbott’s also targeted in litigation alleging failure to warn about risk of infants born prematurely developing necrotizing enterocolitis if fed cow’s milk-based formula.
A National Advertising Division review of claims by LVMH-owned Fenty Skin determined makeup- and dirt-removal representations were substantiated by a study and subject questionnaire, but demo videos from paid endorsers must include disclosures about material connections in accordance with FTC guidelines.
Virus-blocking compound Carragelose, out-licensed to major consumer health players such as Perrigo and Reckitt for use in OTC respiratory nasal sprays, is being adapted for use in the dry eye and allergy categories.
Futura recorded its first notable revenues last year, generated by the launch of its Eroxon erectile dysfunction gel in the UK and Europe, marking the company's transition from an R&D startup to a consumer healthcare player in its own right. To mark the occasion, CEO James Barder outlines a new strategy, with a focus on innovation in sexual wellness, further global partnerships and sustained profitability.
Decisions by “big three” PBMs suggest they’re taking proactive steps to comply with potential HRSA rulemaking to require most health plans, under Affordable Care Act regulations, to cover Opill and other types of OTC birth control without cost sharing.
Australian-heritage Swisse grew its share of China's supplements market in 2023 by delivering tailored products to an increasingly health conscious population.
Sanofi consumer health scientific affairs lead moves to CHPA; change in Bayer’s US consumer health marketing helm; Viatris CCO moves from same post at Moderna; Qnovia expands scientific advisory board; and Powerade powers Girls Inc. scholarships, programs.
Use of Haleon's Otrivin (marketed as Otrivine in some markets) is not just effective at treating nasal congestion during common cold but also “demonstrates significant improvement in overall well-being and quality of life,” a recently published company-funded study shows.
HiSmile appeals NAD recommendation following review on P&G challenge to discontinue ad claims on its website and in social media videos stating peroxide-containing whitening products are “painful” or cause pain, break down and impact gums and teeth, or “damage” gums.
The Independent Beauty Association does not yet have insight into what ingredients will be listed in the US FDA’s proposed rulemaking for cosmetic allergen labeling – due by 29 June – but is optimistic the agency will consult industry when it comes to setting the compliance timeline. Meanwhile, industry stakeholders are pushing for international harmonization in the agency’s cosmetic GMPs rulemaking.
Without FDA regulatory pathway for hemp’s lawful use in supplements and food, some states are imposing more-stringent regulations while others allow sales of intoxicating ingredients not considered controlled substances as hemp derivatives.
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.