Medtech 2018: The Place For Innovation As Value-based Health Care Gains Momentum
Executive Summary
2017 was a watershed year in many respects, politically, economically and commercially for many players in the medtech field. Where will the opportunities lie in 2018, will breakthrough medtech innovation still have a place among providers often riding on fumes when it comes to budgets, and is it all as bad as some would make out?
- Medtech M&A in 2017 continued at the impressively high level of recent years, but no matter how many transactions take place, there are always more candidates large and small in the wings.
- As stakeholders embrace a focus on outcomes, sustainability and system cost reductions, the industry is moving away from fee-for-service and supply push – but not totally, and not as quickly as originally thought.
- Left unaddressed, medtech portfolios eventually become commodity-dominated, but this spells danger for innovators, because innovation is where the growth, the R&D returns and the future of the high-risk medtech industry lies.
- So what? Sharing the total cost of care might not have been in a company's mission statement 30 years ago, but the wording is now indelible across the industry. Forward-looking medtechs will seize the opportunity, as indeed they must, for there are many largely untapped as well as substantially saturated markets available – and they don't tend to shrink.
The value-based health care agenda gains incrementally more currency with each passing month. Medtech companies have long since moved on from "when and if," to "how and whom," as they size up both the opportunity and cost of playing an enhanced role in health care delivery. They must decide whether to commit themselves to a holistic system where efficiency of operational performance, cost reductions, lowering readmissions and patient satisfaction are allied to outcomes improvements.
The technology remains central in this new environment, and innovation paramount. But it is no longer the endgame, rather a stage in the overall process – albeit an essential one. It is an environment where manufacturers, at least, see themselves as partners of the providers in health care delivery. This is indeed a compelling argument in cases such as Johnson & Johnson's CareAdvantage and Medtronic PLC's Integrated Health Solutions programs that aim to develop tailored services and solutions to improve clinical, operational and financial outcomes. (Also see "Options For Medtechs In A Value-Based Care World" - In Vivo, 8 Nov, 2017.)
Those able to take advantage of the shift in health care priorities are building new business models consistent with mandatory bundles like the Centers for Medicare and Medicaid Services' (CMS') Comprehensive Care for Joint Replacement (CJR), and shared services. CMS pulled back somewhat from mandatory bundled concepts in the latter part of 2017, but the move away from fee-for-service remains well underway: up ahead await newer concepts still, such as population health management, which will use data analytics on individuals within population subgroups to improve clinical outcomes and reduce costs; and risk-sharing deals, such as the Aetna/Medtronic agreement of June 2017 (see below), on a broader scale. (Also see "Medtronic’s Deal With Aetna Heralds New Value-Based Era" - In Vivo, 18 Sep, 2017.)
However, while value-based health care is the present and the future, physicians don't see it as tearing up the tarmac, not yet at least. A June 2017 study by Quest Diagnostics Inc.and Inovalon, surveying physicians and health plan executives on the penetration of value-based health care in the US, found that the health care industry continues its systemic shift from a fee-for-service delivery model to a value-based system that aims to deliver and pay for health services based on quality rather than quantity, but that progress is only moderate.
In 2016, a quarter of physicians and health plan executives believed the US had a value-based care system; in 2017 the figure had risen to only 29%, according to the Quest/Inovalon survey. Longer-serving professionals were more likely to consider that fee-for-service continues to dominate health care, but the general view remains that a majority of individuals at the point of care believe that US health care is behind the adoption curve. In its ongoing Global Assessment Initiative with the Economist Intelligence Unit, Medtronic stresses that many countries have a strong political will and are moving in the right direction. Overall, the value-based health care model is being introduced in step-by-step fashion.
That view is shared by the Boston Consulting Group. Interviewed by In Vivo at year-end, health care and medtech specialists at the global management consulting firm said that while up to four-fifths of the US market is talking about risk-based/value-based outcomes, less than 15% of current US payments can be classified as value-based or risk-shared – and even those are still largely fee-for-service plus an incentive, as opposed to a true insurance risk, or population-based payment. The rest remain fee-for-service, for procedure-based reimbursement.
So it's not an overnight transition, to say the least, but it is the direction of travel. US ACOs using value-based concepts are finding that more of them (50%) saved money in the third year of participation than in the first (33%), according to a recent Avalere Health webinar. The notion that users are seeing more benefits of value-based health care is borne out in the Quest/Inovalon survey finding (see above) that 70% of health plan executives had noticed progress in the alignment of health plans and providers of health care. Although fewer than half of physicians were of this view, in all, over eight in 10 of the combined survey group agreed on the pressing need for alignment between payers and providers to achieve value-based care.
Aetna And Medtronic Show The Way
The non-exclusive, outcomes-based deal between payer Aetna and Medtronic is designed to support the transition of patients with type 1 or type 2 diabetes away from multiple daily insulin injections and toward Medtronic's MiniMed 530G, 630G and 670G insulin pumps. These devices feature SmartGuard technology, which prevents hypoglycemia (which can stop patients from reaching HbA1c goals). The health outcomes in these patients are measured, and part of Medtronic’s reimbursement will depend on the achievement of pre-agreed clinical improvement thresholds.
The drivers of this won't be the government, CMS or particularly manufacturers, but provider systems – doctors and large health systems that can provide better outcomes quality than other systems – and large commercial payers. Aetna Inc., which has size and influence and an incentive to shift to a value-based world, has shown the way. The story of its mid-2017 deal with Medtronic relating to the supply of insulin pumps was one of In Vivo's most-read features of last year, partly because it pointed to the shape of things to come in terms of supply deals for the medtech sector as a whole. (Also see "Medtronic’s Deal With Aetna Heralds New Value-Based Era" - In Vivo, 18 Sep, 2017.) (Also see box, Aetna And Medtronic Show The Way.)
As long ago as 2012, the American Diabetes Association put the cost of diabetes to the US at $245 billion, with $176 billion in direct costs (including $90 billion in hospital inpatient and nursing home expenses and $7 billion in emergency care) and $69 billion in indirect costs/lost productivity. The figure could now be as high as $322 billion. The question for manufacturers, as they ultimately help to bring down provider costs, is how they will be sustainably rewarded, not just in diabetes, but in AF and HF and indeed all other long-term conditions, where the value-based agenda will have most leverage.
The Trump administration, backed by the Republican-led Congress, continues its drive to repeal and replace the Affordable Care Act, which went live just over seven years ago. This uncertainty over the ACA's future may destabilize some facets of health care, but the transition to value-based care is not one of them. Indeed, over 80% of physicians and health plan executives say they believe the transition to value-based care will continue, regardless of legislative reforms spearheaded by the federal government.
The ACA is proving hard to withdraw entirely due to the GOP's wafer-thin Senate majority that includes John McCain and two other Republicans opposed to ACA repeal, and the total opposition of the Democrats. But what could happen to the ACA in 2018? US President Donald Trump has sought to save face after successive repeal failures in 2017 by claiming that the ACA was “essentially repealed” after eliminating the individual mandate in his successful tax reform bill; the mandate required those who opt out of coverage to pay a penalty. It means that Republicans have secured one significant legislative victory against Obamacare, but other aspects of the ACA may still be targeted (Medicaid expansion being the biggest). Repeal will still be on the table in 2018.
The comprehensive tax reform was arguably the president's only major victory in a roller coaster first year in office; it means among other things that corporations will see their headline income tax rate drop from 35% to 21%, bringing the US more in line with the average rate in the developed world.
The Dreaded US Sales Tax
But will the ACA's 2.3% device sales excise tax return? It was due to do so this January 1, the temporary moratorium in force since 2015 having ended at the turn of the year. Industry was getting increasingly agitated at the prospect of a reinstatement of what it has called a job-killing tax as the clock ran down late in the year. Under the legislation, the total cost to the medical device industry would be roughly $20 billion over 10 years. A House bill late in the year, the Bipartisan Market Stabilization and Innovation Act of 2017, included tax repeal wording.
This single issue dominated much of AdvaMed's US market support efforts in the 2013–15 period, when the tax was being levied, but as US equity analyst with Jefferies Healthcare Raj Denhoy says, nobody really expects the tax to return permanently. (See online sidebar, "Tough At The Top: Raj Denhoy On The Drive For Growth In A Maturing Medtech industry.")
Nevertheless, the US industry is doing its work anyway, arguing that the tax penalizes US companies unfairly. Medical device trade association AdvaMed president and CEO Scott Whitaker wrote to the president in mid-December, reminding him of the tax's negative impact on medical innovation, a resulting loss of 29,000 jobs (according to the US Department of Commerce), reduced R&D and slowed capital expansion. Whitaker was seeking swift action before the bi-weekly January payments were due to kick in – with any potential refunds not being made by the IRS until the end of the year.
Still A Place For Innovation?
Does this really affect medtech innovation, given that innovation is a moving feast in a fast-changing industry? Johnson & Johnson, for one, says that its continued growth and success depends on its ability to innovate products and services that address the evolving health care needs of patients, providers and consumers. New products introduced within the past five years accounted for around 22% of its 2016 sales, for example.
Innovation, in fact, still means everything. Boston Scientific Corp.is not alone warning that any impediment to launching new and enhanced products would negatively impact the group's performance. Boston, Stryker Corp. and Medtronic have all made efforts to innovate away from the commodity element of their sales mix, and over the past five years have all managed it to below 50%.
The German industry association, BVMed, ever seeking to underline the totally different marketing models of medtech versus pharma, says that 33% of national medtech sales in Europe's largest medtech market are made with products that are no more than three years old. Its point is that innovation is as crucial a differentiator as ever, even if the real innovation up ahead will be in the delivery paradigm and keeping patients out of the system. And if they're in the system, treating them as efficiently as possible, with reproducible methods using robotics and leaning on artificial intelligence and machine learning wherever possible.
Given the higher stakes, targeted and applied innovation is flourishing. Cleveland Clinic's annual list of the medical innovations that could potentially transform the sector shows how medtech innovators will be pushing back the boundaries of patient care in 2018. It's a snapshot of areas where R&D efforts are meeting demand pull – not the supply push that was once the holy grail for manufacturers. The fields where innovation will help global health care break new ground this year, according to Cleveland, include:
- Diabetes Control: A hybrid closed-loop insulin delivery system that helps make type 1 diabetes more manageable by enabling direct communication between the continuous glucose monitoring device and insulin pump to stabilize blood glucose is set for mainstream use. In 2018, with more patients likely to demand the technology, more insurers will reimburse for it. It is also predicted that this will accelerate a type 2 diabetes product.
- Neuromodulation: Another innovation tipped to disrupt the market in 2018 is an implant that delivers stimulation that opens key airway muscles during sleep. Although sleep apnea impacts 21 million Americans, more than 40% of sufferers reportedly dislike the idea of the continuous positive airway pressure (CPAP) device. But the implant, controlled by a remote or wearable patch, helps to synchronize the intake of air with the action of the tongue using a breathing sensor and a stimulation lead powered by a battery.
- Cancer therapy: Targeted therapies will become widely used to treat breast cancer in 2018. PARP inhibitors for patients with specific mutations in BRCA1 or BRCA2, and novel CD K 4/6 inhibitors for ER-Positive/HER-2-negative breast cancer are having positive outcomes in clinical trials. Novel HER-2 targeted agents continue to show benefit in this subgroup of HER-2-positive patients. These studies point to an increasing survival rate, and perhaps the eventual end of chemotherapy for a significant population of breast cancer patients. That said, the current mechanisms – hormone therapy, chemotherapy and radiation – are still seen as valuable options for prolonging life, however they are often not enough to keep cancer at bay.
- Chemotherapy hair loss: Loss of hair post chemo can have a major effect on women. A new FDA- approved technology, “scalp cooling,” which reduces the temperature of the scalp a few degrees during and after chemo, has been shown to be highly effective for preserving hair in women receiving chemotherapy for early-stage breast cancer.
- Gene therapy for retinal diseases: In mid-December the FDA approved Spark Therapeutics Inc.'s gene therapy Luxturna (voretigene neparvovec-rzyl), for inherited retinal diseases, which should provide visual function improvements in some Leber congenital amaurosis and retinitis pigmentosa patients. Experts believe the approval could lead to more gene therapies getting orphan drug and breakthrough status.
No Longer Just "Product-Focused" Solutions
Cleveland's selected solutions for 2018 are not just product-focused, however. Mirroring the change in perceptions of what constitutes health care innovation in this second decade of the millennium, its list of innovations includes several broader concepts that assist health care delivery in all settings.
- One is telehealth, finally making a reality of extending video and sensor monitoring platforms and other distance health concepts to patients' homes. Increasing connectivity through mobile technology has seen hospitals get ready for widespread adoption in 2018: 90% of US health care executives are reportedly building a telehealth programs to cater to 7 million patient users in 2018.
- Similarly, another long-talked of goal, centralized monitoring of inpatients, is becoming the realistic and efficient alternative to constant attention by bedside caregivers, where the risk is that they may become desensitized to patient needs/alarms among the noise on the ward/unit. Instead, off-site personnel using sensors and high-definition cameras can monitor vital signs, and establish thresholds for when on-site intervention is required.
- And a third is the implementation of fast-track or ERAS (Enhanced Recovery After Surgery) methods for patients post-surgery. A recent protocol that permits patients to eat before surgery, limits opioids by prescribing alternate medications and encourages regular walking has been shown to both reduce complication rates and speed recovery. The spin-offs include fewer blood clots; less nausea, infection and muscle atrophy; and shorter hospital stays. Such protocols are expected to gain further ground in 2018.
Product pipelines have always driven investment trends in medtech, says BMO Capital Markets, which predicts this same trend in 2018. It believes the evolving diabetes landscape, transcatheter mitral valve repair/replacement market, transcatheter aortic valves, minimally invasive glaucoma surgery (MIGS) and robotic-assisted surgery applications (large joints, spine and surgery) will all merit closer investor attention in 2018. These will help expand the global market (including IVDs, dental and health care IT products to around half a trillion dollars by 2022, according to Jefferies Healthcare). (See Exhibit 1.)
Exhibit 1
Major Medtech Markets Worldwide – 2022 Prediction
Over the coming five years, CAGR averaging 5% is expected to be achieved in the major medtech markets, including IVDs – the largest individual medtech sub-segment both now and in five years' time, when a market value of over $70 billion is predicted. The leading medical device sub-segments are forecast to be worth the following values in 2022.
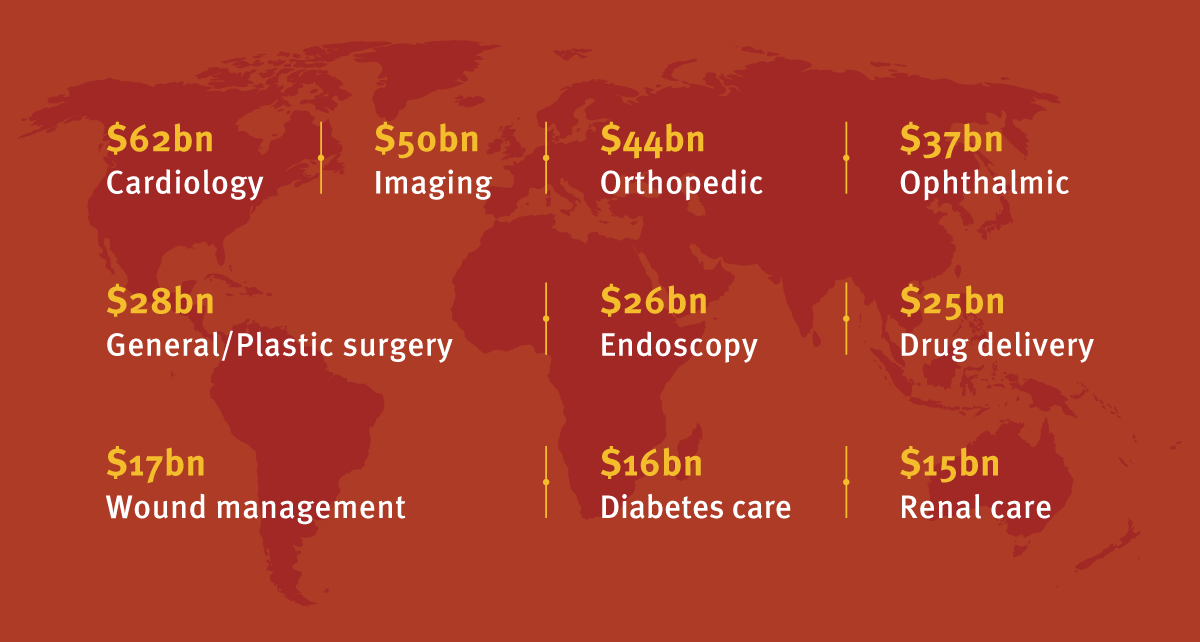
Jefferies Healthcare
Avalere Health adds that a lot of innovation is happening in the digital health space, and also praises the potential for good, in terms of encouraging innovation, of the US' 21st Century Cures Act and FDA commissioner Scott Gottlieb, MD's, risk-based approach to regulation. (See online sidebar, "Gottlieb's FDA Lights A Fire Under Medtech Policy Activity But Reimbursement Challenges Remain.") Says Brian Chapman, head of ZS' Global Medtech Consulting practice, "Not every 'innovation' is worth having, but one of my wishes for 2018 is flexibility and innovation in how we pay for digital health." (Also see "Resilience Is Key For Medtechs Facing Provider And Payer Flux In 2018" - In Vivo, 23 Jan, 2018.)
And there are plenty of areas of substantially unmet need that await greater innovator attention, including depression, deafness, dementia, blindness, peripheral vascular disease, obesity, stroke and aneurysm.
The Drive For M&A Will Continue At A Faster Pace
M&A plays are a quicker if more expensive way of acquiring innovation. The number of $1 billion-plus medtech deals announced in 2017 and reported by In Vivo and sister publication Medtech Insight hit a round 10. (See Exhibit 2.) The standout Becton Dickinson & Co./CR Bard Inc.deal continued the trend of the creation of larger, diversified companies that can better leverage hospital relationships seen in recent years. According to BMO Capital Markets, the outstanding potential candidates for such a merger include Smith & Nephew PLC, Boston Scientific and Edwards Lifesciences Corp.
Exhibit 2
The Billion-Dollar Medtech Deals Of 2017
|
Acquirer |
Target |
Field |
Consideration |
Announced |
|
Becton Dickinson |
CR Bard |
Medical devices |
$24bn |
April 23 |
|
Rationale: Creation of a highly differentiated medtech uniquely positioned to improve the process of care and the treatment of disease for patients and providers. |
||||
|
Cardinal Health |
Medtronic's Patient Monitoring and Recovery division |
Patient monitoring |
$6.1bn |
April 18 |
|
Expanded product offering, addition of well-established brands that are a "natural fit." |
||||
|
Allergan |
Zeltiq Aesthetics |
Medical aesthetics |
$2.475bn |
Feb. 13 |
|
Immediately accretive and enhances Allergan's global medical aesthetics portfolio in a $4 billion market. |
||||
|
Philips Healthcare (Netherlands) |
Spectranetics |
Imaging – laser atherectomy catheters for both coronary and peripheral indications |
$2bn |
June 28 |
|
Strengthens Philips' image-guided therapy business in a €6bn market. |
||||
|
Fresenius Medical Care (Germany) |
NxStage Medical |
Urology – dialysis market |
$2bn |
Aug. 7 |
|
Supports core business growth with the offering of innovation, better clinical outcomes (through Care Coordination) and improvement of the patient experience. |
||||
|
Hologic |
Cynosure |
Medical aesthetics |
$1.65bn |
Feb. 15 |
|
Gives Hologic entry into one of the fastest-growing segments in medtech; expands Cynosure's customer reach. |
||||
|
Shanghai Pharmaceuticals(China) |
Cardinal Health China |
Cardinal Health's pharmaceutical and medical products business in China |
$1.2bn |
Nov. 14 |
|
Greater distribution reach in China. |
||||
|
Teleflex |
NeoTract |
Urological medical devices – minimally invasive tech for treating lower urinary tract symptoms due to BPH |
$1.1bn |
May 9 |
|
Boosts Teleflex’s drive for mid-single digit constant currency sales growth for the next several years with products that have long product life cycles that benefit from patent protection, and demonstrate superior clinical benefit while providing cost benefits to hospitals. |
||||
|
CooperSurgical |
Product asset |
Paragard intrauterine copper contraceptive device from Teva (Israel) |
$1.1bn |
Sept. 12 |
|
Non-core Teva product, makes Cooper only company with an IUD on the US market that is hormone-free, long-lasting and reversible. |
||||
|
Integra LifeSciences |
Codman Neuro |
Neurology |
$1.045bn |
Feb. 16 |
|
Expands Integra's global leadership with the addition of a broad medical device portfolio in the neurosurgery market. |
||||
Note: US unless stated.
In Vivo research
However, 2018 may well see the renewed prominence of bolt-on acquisitions for technology additions and growth. Products offering a competitive advantage in the non-commodity bracket will be picked off to bulk out sales mixes, and the range of buyers could include Baxter International Inc., Boston Scientific and Johnson & Johnson, and the sellers might include, among others, Glaukos Corp., NuVasive Inc., Wright Medical Group NVand chronic pain relief therapy provider Nevro Corp.
Other Issues To Note In 2018
EU Regulatory Change Is The Market Access Talking Point Bar None
The biggest regulatory change for many years in the leading medtech markets globally finally went live in 2017: the EU Medical Device Regulation and its sister IVD Regulation became effective in May, subject to three- (MDR) and five- (IVDR) year transition periods. When the MDR enters into full force, it will impose significant additional premarket clinical data requirements, higher-level scrutiny and more stringent postmarket requirements. It will eventually introduce Unique Device Identification (UDI) among many other changes. (Also see "Medtechs Must Act Fast On New EU Regulations Or Face Gridlock" - In Vivo, 26 Apr, 2017.)
ConCeplus says the transition to the EU MDR is the greatest medtech industry challenge for the coming 3–5 years.
There are significant capacity and timing concerns surrounding the new MDR. The transition to the EU MDR is the greatest medtech industry challenge for the coming 3–5 years, according to Swiss-based think tank ConCeplus' October 2017 LimedEx Index report. It puts the overall ecosystem compliance cost at $18 billion in the coming three years, and notes that it represents an involuntary shift to pharma-style compliance (evidently much to BVMed's displeasure), which will affect R&D budgets and thus the ability to innovate.
The average EBIT impact across manufacturers serving the EU will be –4%. Resources are already thin enough in medtech, but to cope with the changes, it is considered that companies will need to hire an aggregate 31,000 extra FTE staff for their governmental and regulatory affairs departments. Who will pay for all this, and how, are questions that apparently no one has been tempted to tackle with any great alacrity. There is no choice but to comply; the advice remains, as in the previous 18+ months, don't leave it to the last minute and risk your products being EU-market ineligible.
Brexit Is A Storm In A Medtech Teacup
The UK – a soon to be ex-EU member state against most conventional wisdom and advice – has the additional burden of hoping it can use the EU's medtech regulations while being outside the club. The loss of the UK Medicines and Healthcare Products Regulatory Agency's (MHRA's) technical input on EU regulatory matters, as the agency's balanced, free-market, company-friendly thinking, will impede the system, possibly immensely. Brexit is a matter of deep regret for many; however, the "uncertainty" – surely the Word of the Year in both the UK the US for the second time running – should be over for the UK by the end of 2020, when the EU-imposed transition period for the UK's exit ends. (Also see "UK Medtech One Year Post-Brexit Vote: Still In The Land Of Uncertainty" - In Vivo, 30 Jun, 2017.)
The UK industry association (ABHI) is preparing contingencies for possible loss of market access, and among its many planning and support activities has recently joined the Global Medical Technology Alliance (GMTA). Many companies wonder about the true impact on their business, and should at last know more when EU-UK trade deal negotiations start, which the EU says will not be until March 2018 at the earliest.
For now, medtech is taking a typically balanced view. LivaNova PLCCEO Damien McDonald told In Vivo that Brexit would not have a material effect on business, and would not encourage it to move its HQ out of the UK. (Also see "True To Its Word, LivaNova Sheds CRM – To MicroPort" - In Vivo, 4 Dec, 2017.) BioMerieux Inc. says that the UK (representing some 3% of its global sales in 2016) leaving the EU would not present a risk that could have a significant impact on its accounts. In general, medtech industry players outside the UK tend to view the whole Brexit episode with mild confusion.
Dedicated Global Regulatory Systems Are The New Black
The MHRA should soon find an enhanced global role for itself, and will surely bring its skills and experience to bear in matters of the International Medical Device Regulators Forum (IMDRF) and global medtech regulatory harmonization.
Indeed, much is happening elsewhere, in Asia-Pacific especially, with, for example, a new conformity assessment system bedding in Malaysia, a brand-new medtech regulatory system (deferred to January 1, 2019) for Vietnam, smart regulation being prioritized in Singapore, a new IVD regulation in the Philippines and the Asean bloc's regulatory "harmonization" creating a new, potentially industry-friendly system.
Ukraine (a new EU-aligned system based on the EU's three Medical Device Directives), South Africa (the long-awaited device regulatory system finally starts up in 2017) and Russia and the five member-state Eurasian Economic Union are also among those moving into hitherto uncharted territory, strengthening their national regulatory systems and/or harmonizing, in moves that will pay dividends in terms of patient care, quality and support for innovation.
Final Thoughts – Thinking Differently
Global medtechs in 2018 know that health care spending in its current form is unsustainable, and that they must be alive to the shifts in incentives and a realignment of players in health care delivery. Amid the consolidation of and mergers among large hospital systems, buyers will be keener to do business with fewer, broader medtech clients, hence some of the rationale behind the Becton Dickinson and Bard merger, in the example of several of similar or larger magnitude in the past five years.
Medtechs are having to shoulder a new responsibility – sharing the total cost of care. And as the commoditized portion of the typical medtech company's product mix naturally continues to expand, the pressure on that company to offer value with solutions that maximize outcomes means that the premium on true innovation is as high as it's ever been.
It is true that changes in delivery models have forced the industry to think differently, but on the other hand medtech markets remain vast, and many are under-penetrated. Medtech is, in fact, in pretty good shape. Rather than declining in importance, some aspects of "innovation" have simply become different to what the industry has been accustomed to. The culture shift continues in 2018.
