Nonprofit Pharma Targets Making Naloxone Available OTC, Trimming Price
This article was originally published in The Pink Sheet
Executive Summary
Nonprofit Harm Reduction Therapeutics targets an OTC switch of naloxone, an opioid antagonist used to reverse an opioid overdose. It aims to make the product available at low cost in 110,000 retail locations nationwide.
Harm Reduction Therapeutics Inc. is taking a nonprofit approach to a naloxone OTC switch with a goal of offering it in “every drug store in America" to help prevent opioid-related deaths, says founder and CEO Michael Hufford.
Hufford said the Pittsburgh firm seeks to raise $10m funding to conduct research for an OTC switch application and eventually launch a naloxone product at a low cost with broad availability in some 110,000 retail locations, including 35,000 drug stores as well as gas stations and convenience stores. It also seeks to make the drug widely available to first responders including emergency medical technicians, law enforcement officers and firefighters. Harm Reduction Therapeutics is registered as a tax exempt nonprofit under section 501(c)(3) of the US tax code.
Response To FDA Push
Harm Reduction Therapies targeted a naloxone OTC switch after FDA Center for Drug Evaluation and Research officials said at a May 2017 consumer health regulatory conference that the agency had funded labeling studies about OTC use, assessing whether pictograms could show “how to safely use naloxone, including when it is appropriate to purchase it and how to use it in an emergency." Division of Nonprescription Drug Products Director Theresa Michele said the research could spur industry interest in resolving unmet public health need and encouraged manufacturers to correspond with the agency regarding potential switch applications. (Also see "FDA's OTC Naloxone Study Is A Starting Point For Other Switches, Not A Roadmap" - Pink Sheet, 16 May, 2017.)
Hufford said FDA's effort to prompt switch applications is “remarkable” and “unprecedented” and struck a chord with him.
“As we started talking about it and looking into it, we got more and more frustrated with the status quo that someone needed to do something about it and that someone should be us.”
Naloxone is an opioid antagonist indicated for complete or partial reversal of opioid overdose, including respiratory depression. An OTC version would be an intranasal product, the format emergency services personnel prefer to use for opioid overdoses, the cause of 100 deaths in the US per day, Hufford said in an interview.
“I’ve been doing drug development for 20 years and fundamentally, people’s lives are being lost because of a combination of inadequate access and excessive cost” for naloxone, said Hufford, who has co-founded multiple pharma, medical device and mobile health companies, led pharmaceutical development teams through FDA approvals and assisted in Rx-to-OTC switches.
Multiple injectable naloxone generics are available by prescription in the US for use by medical professionals and emergency personnel; two other Rx products in some states are also being made available to consumers: the Evzio auto-injector currently marketed by kaleo Inc. and Adapt Pharma Ltd.’s Narcan nasal spray. The ingredient originally was approved by FDA in 1971 and went off patent in 1985. (Also see "Amphastar's Naloxone Nasal Spray Delayed; User Human Factors Study Among FDA Concerns" - Pink Sheet, 21 Feb, 2017.)
In October, President Trump declared the epidemic of people abusing opioid drugs prescribed for pain or using heroin instead of legal substances a national public health emergency and directed all executive agencies to use “every appropriate emergency authority” to stem the crisis. (Also see "Declaring Opioid Emergency, Trump Touts FDA Actions, NIH-Industry Partnerships" - Pink Sheet, 26 Oct, 2017.)
Naloxone Price Up, Heroin Down
Hufford says he is "sickened” that the opioid abuse crisis has spiked naloxone prices. He noted in a recent blog on the firm's website that a nasal spray or auto-injector for use by nonmedical personnel costs $110 and $4,000 as the prices have increased 95% to 500% over the past few years.

Michael Hufford: "people’s lives are being lost because of a combination of inadequate access and excessive cost” of naloxone.
Conversely, as naloxone's cost has increased and its availability remains largely unchanged, the cost of heroin has dropped amid growing availability. “What’s happened to heroin versus naloxone, they are tragic mirror images of each other,” he said.
Some states have made Evzio and Narcan available nonprescription from pharmacies, or through pharmacists' prescriptions. In addition to local pharmacies, national drug chains are offering less stringent access to the products where allowed (see table below for overview of standing order policies and availability without prescription in individual states).
But pharmacy sales are ”completely insufficient to address the epidemic," Hufford said. The drug still is not inexpensive – $110 for a single nasal spray in the Pittsburgh area, he said.
Access to naloxone still is limited in behind-the-counter distribution because “you still have to interact with the pharmacist, you still have to know what it is in the first place and you are left paying retail or if you have insurance, the co-pay,” he said.
Additionally, with the drug needed in emergencies, broader access in venues such as service stations and convenience stores would make it easier to obtain than from a pharmacist.
“Statistically, the most likely person to be present at an overdose is another opioid user, and they may be hesitant to interact with a pharmacist in that situation. So you want them to have it in case they need it to rescue a friend or family member,” Hufford said.
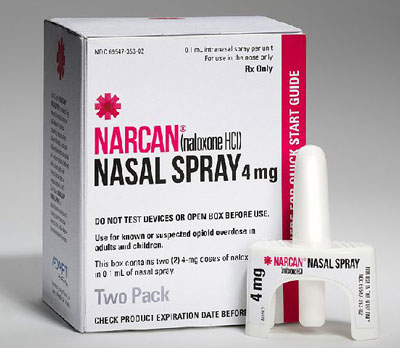
Adapt Pharma’s Narcan nasal spray, above, and the Evzio auto-injector marketed by kaleo, below, are deemed Rx by FDA but are available nonprescription from pharmacists in some states.
Hufford said Harm Reduction Therapeutics plans to offer OTC naloxone at a price that will cover manufacturing costs, “functionally at breakeven.”
“My goal, to the extent to which we are successfully fundraising, it would be possible to actually sell [the OTC drug] into the retail supply chain at or below cost. The extent to which you have already covered your cost and raise money above that, you would actually use some of that money to offset manufacturing cost.”
A nonprofit pharma firm is "an oxymoron in lay people’s minds, and the antithesis of how they think of the pharmaceutical industry," Hufford said. Consumers view firms as driven by profit even though drug research and development is costly, he said.
“This is a small opportunity to rehabilitate our image and renew that social contract that when things go off patent and are available in generic form and are life-saving, we have a responsibility to make sure we are fixing the problems of cost and access.”
Public Awareness Key
A naloxone OTC switch's success will be only as good as consumers’ awareness it's available. Harm Reduction Therapeutics is in talks with a group about a public education campaign to inform consumers in much the same way other OTC switch ad campaigns do.
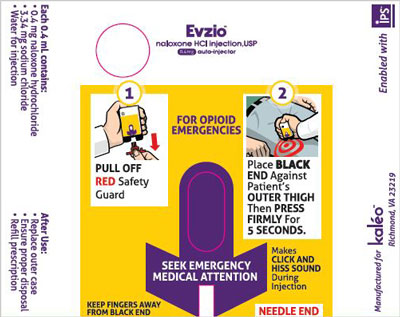
Evzio labeling includes a pictogram in usage instructions, a format FDA has studied for label comprehension for an OTC naloxone.
Hufford said he “would love to” have support from the Consumer Healthcare Products Association trade group “We have had some informal communication with folks there to let them know we are doing this, but I haven’t asked for support at this point. So, when the time is right we would absolutely reach out to CHPA to make use of their tremendous public education networks and capabilities.”
CHPA has conducted campaigns to educate consumers on OTC use and disposal and has ongoing work with several counties in the US on proper disposal to counter the momentum from local governments and one state to require the drug industry to pay for takeback programs. (Also see "CHPA Optimistic Voluntary Drug Takeback Efforts Will Stall Spread Of Mandates" - Pink Sheet, 6 Jan, 2017.)
Standing Orders, Third-Parties And Pharmacists' Prescriptions
Few states currently have no program to make naloxone available without a doctor's prescription, though the drug remains Rx-only at FDA.
National Association of State Pharmacy Associations
From the editors of the Tan Sheet.
