FSMA Supplier Verification Rule Affects Finished Supplement Importers
This article was originally published in The Tan Sheet
Executive Summary
Proposed FSMA rules would subject imported food products, including supplements, to the same safety standards as domestically produced food. The proposals are reminders that although supplement manufacturing is exempt from FSMA provisions redundant to GMP requirements, the act adds regulatory burdens for some firms.
FDA’s food safety foreign supplier verification program proposed rule adds a regulatory burden for importers of finished dietary supplement products, while leaving ingredient importers exempt from the brunt of the regulation.
The proposed rule, issued under the Food Safety Modernization Act, would subject imported food products, including supplement ingredients and finished products, to the same safety standards as domestically produced food (Also see "Supplement Ingredient Suppliers Should Prepare For Food HACCP Regs" - Pink Sheet, 9 May, 2011.).
FDA published both the FSVP proposal and a separate proposed rule for FSMA’s third-party auditor certifications provision in the July 29 Federal Register (see story below, “Accrediting Auditor Accreditation Groups”).
The deadline for comments is Nov. 26. The agency plans three public meetings to discuss the rules, with the first scheduled for Sept. 19-20 in Washington, according to an Aug. 16 notice.
For supplement industry stakeholders, the FSVP proposed rule is a reminder that even though supplement manufacturing operations are exempt from FSMA provisions that would be redundant to supplement GMP requirements, the act impacts some firms in the space.
“As these kinds of rules are put in place, people are going to start to realize, ‘I do have to think about this. The quality practitioners in the company will have to account for this,’” said Duffy MacKay, the Council for Responsible Nutrition’s VP for scientific and regulatory affairs.
“These proposed rules are not simple and they’re not minor. They’re big,” said Cara Welch, the Natural Product Association’s senior VP for scientific and regulatory affairs.
“Even though dietary supplements are excluded for the most part, there still are extra requirements,” she said.
FDA says imported food products reach the U.S. from about 150 countries and account for about 15% of the food supply, including about 50% of fresh fruits and 20% of fresh vegetables.
Supplement industry stakeholders estimate the percentage of imported materials that firms use likely is much higher, with some ingredients available only from other countries and with own-label distributors commonly relying entirely on imported finished products.
However, FDA currently has limited authority to ensure the safety of imported food and inspects less than 2% of all imported food products.
“Rather than relying primarily on FDA investigators at the ports to detect and respond to food safety problems, importers would, for the first time, be held accountable for verifying, in a manner transparent to the FDA, that the food they import is safe,” said Michael Taylor, FDA’s deputy commissioner for foods and veterinary medicine.
Compliance will cost the food and supplement industries $400 million to $500 million, Taylor noted in a June 26 media briefing on the proposals.
FDA explains in the FSVP proposed rule notice that importers always have been barred from bringing adulterated products into the U.S. and many importers already conduct activities to verify the safety of products.
However, “establishing and following FSVPs will necessitate changes to the operations of many importers, especially those that have not previously conducted significant verification activities,” the agency says.
FSVP Hits Finished Product, Ingredient Importers
Under FSMA, firms that import supplement ingredients and that establish and verify those ingredients’ compliance with supplement GMP requirements for identity, packaging and labeling are exempted from most FSVP requirements (Also see "Supplier Verification Rule Increases Import Safety Burden" - Pink Sheet, 25 Feb, 2013.).
FDA says when an ingredient importer is subject to supplement GMPs, the firm’s FSVP requirements are limited to maintaining a list of foreign suppliers, ensuring proper importer identification at entry to the U.S. and maintaining import records.
The supply chain security achieved in supplement GMP compliance is redundant to most elements of FSVP. In the notice, FDA points out that imported supplement ingredients will be subjected to further processing by U.S. manufacturers.
However, according to the proposed rule, importers of finished supplement products – packaged and labeled dietary supplements that are not subject to further processing – are required to comply with most FSVP requirements.
Importers of finished supplement products must verify that suppliers of the ingredients are in compliance with U.S. supplement GMP regulations. This means they would not have to evaluate the hazards reasonably likely to occur, as other food product importers must do, because the supplement GMP regulations effectively address the control of relevant hazards by including provisions encompassing all aspects of supplement production, FDA says (see diagram below).
Dietary Supplement Firms' FSVP Requirements
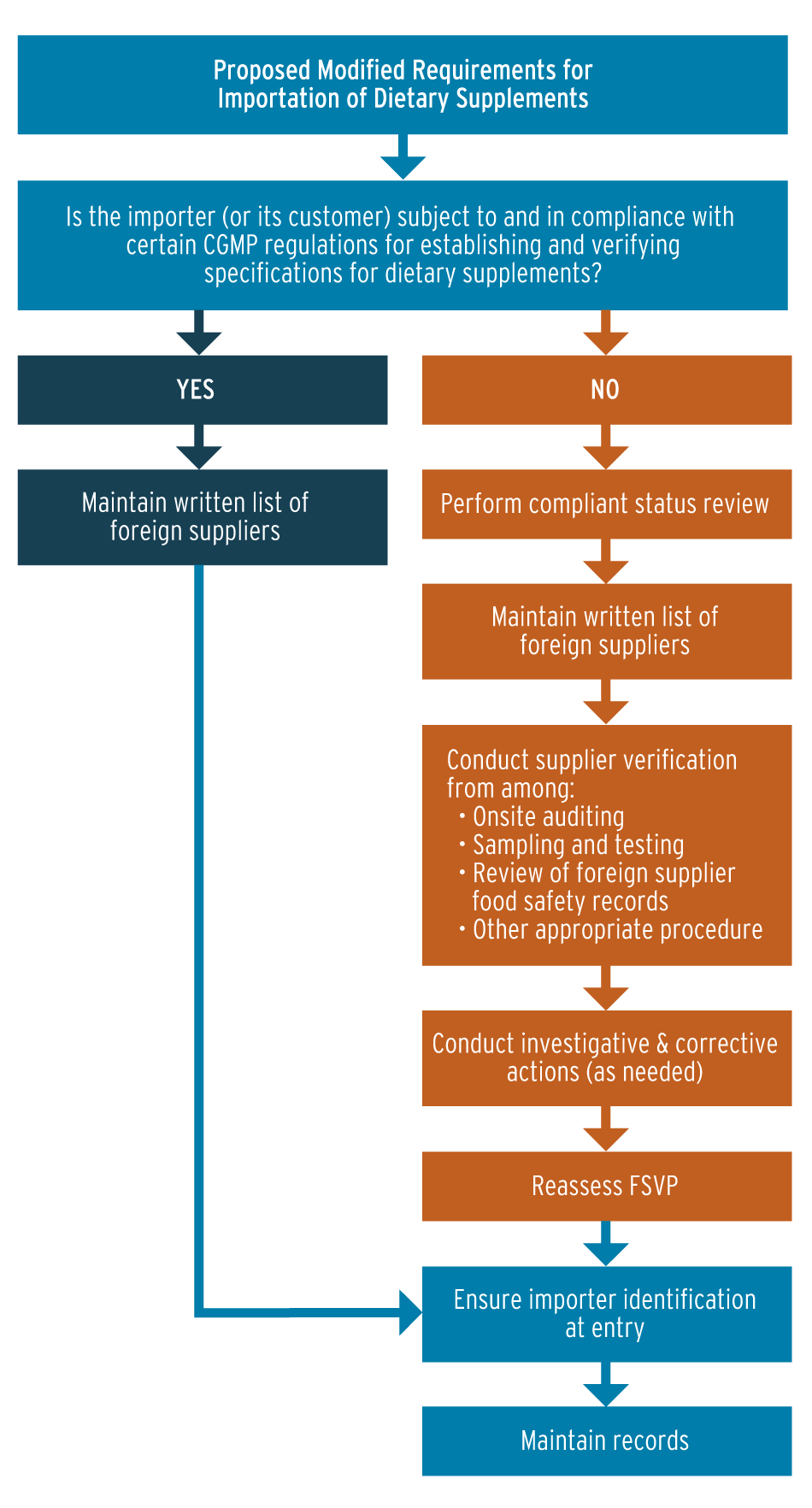
FDA lays out supplement firms' regulatory pathways for compliance with the foreign supplier verification program under FSMA.
FDA proposed rule
More Visibility Upstream
The focal point of supplement firms’ additional regulatory burden from foreign supplier verifications is in increasing their visibility into upstream steps in the supply chain.
CRN’s MacKay said while supplement manufacturers importing ingredients should already be on top of their suppliers as part of their GMP compliance efforts, the FSMA rule extends the same requirements to businesses that import and then sell ingredients and to firms that import finished supplement products.
“What this is basically saying is that you as the importer have to have some checks and balances to make sure that stuff coming into the country is made in compliance with” U.S. supplement GMP regulations, he said.
“If you’re having someone contract for you, it doesn’t mean that you’re forgiven from making sure that stuff is made in compliance,” he added.
NPA’s Welch pointed out that importers will have to comply with different sets of regulations when they supply the same ingredient to supplement manufacturers and food producers. While supplement GMPs cover the ingredient’s use in supplements, the hazard analysis and critical control point requirements imposed by FSMA will apply to its use in food.
“We know that there are a fair number of companies and facilities that produce dietary supplements as well as foods so they would have to be complaint with both. It’s not an either/or here. For every product that is a food, you have to be compliant with preventive requirements; for every product that is a dietary supplement, you have to comply with dietary supplement GMPs,” she said.
MacKay said proposed FSMA rules in some ways parallel the Standardized Information on Dietary Ingredients Work Group voluntary vendor qualification guideline that CRN, the Consumer Healthcare Products Association and the United Natural Products Alliance published in 2012.
“A lot of the principles espoused in that document are somewhat echoed in this, that you do have to do a fair amount of due diligence to qualify suppliers, which then reduces risk for bad things happening,” he said.
Although the American Herbal Products Association and the Natural Products Association initially participated in the SIDI Work Group, those two trade groups left the process before the guideline’s completion. They disagreed with omitting best practices from the guideline (Also see "Supplier Qualification Guideline Touts Flexibility, Defers Best Practices" - Pink Sheet, 23 Apr, 2012.).
CRN and NPA plan to submit comments on the proposed rule, and NPA will consider developing a training program for compliance with the FSMA rules similar to a GMP certification program it offers.
“Once we have a better idea of the true effect and then, of course moving onto when it’s implemented, we hope to work it into our education program, making sure companies are aware of the additional or new regulations,” Welch said.
“It’s been a real helpful resource for GMP dietary supplement facilities. If we can have that extra resource for dietary supplement suppliers or supplement manufacturers that also produce food, we want to do it.”
|
Accrediting Auditor Accreditation Groups |
|
FDA’s proposed rule sets eligibility requirements for accreditation and says that a third-party auditor can be a foreign government or agency or other entity that meets FDA standards for legal authority, competency and capacity, impartiality/objectivity, quality assurance and records procedures (Also see "FSMA Supplier Verification Rule Close, But Third-Party Accreditation Stalls" - Pink Sheet, 30 Apr, 2012.). Accredited third-party auditors would audit and issue certifications for foreign facilities and would be required to: The proposed rule also sets similar eligibility requirements for accreditation groups, which can be foreign governments or agencies or private entities that meet FDA standards. It would require accreditation groups to assess third-party auditors and monitor their performance, and notify FDA of any changes or denials of accreditation. In a separate notice, FDA will issue draft model accreditation standards to specify qualifications a certification body must have to qualify for accreditation, such as the minimum requirements for education and experience for third-party auditors and their audit agents. The agency will open a docket and accept comments when the draft model is published. FDA would monitor these systems and could revoke an accreditation body’s recognition or withdraw an auditor’s accreditation for good cause. FDA will use third-party auditor certifications to allow expedited review and entry to the U.S. of food and supplement products and ingredients manufactured in certified facilities. Additionally, the agency can require inspection by an accredited third-party auditor as a condition for the entry of products FDA has determined to be potential food safety risks. FDA intends to expedite implementation of the accreditation and third-party auditor programs after publication of the final rule and the final model accreditation standards. Accreditation bodies could apply for recognition when the program goes into effect and auditors could seek accreditation when FDA-recognized accreditation groups begin accepting applications.
|
