OTC DFL ‘Remains Static, Non-Customizable Tool’ As US FDA Lifts Curtain On Potential Changes
Executive Summary
Webinar on DFL requirements was a good launch toward potential changes, says CDER Office of Nonprescription Drugs Director Theresa Michele, "This is a great opportunity for us. I think we are on the cusp of a lot of neat things in the OTC marketplace."
Drug Facts labels required for OTC drugs available in the US have been a success but leave a lot of room for improvement, according to a consensus of Food and Drug Administration officials and drug labeling experts.
“Having been in the marketplace for 20 years, the remarkable safety profile of OTC drugs using the DFL format I think it speaks for itself,” said Jesse Catlin, a California State University, Sacramento, associate professor, during an FDA webinar on 9 June.
“While it is important to recognize what I would argue is overall success of DFL, it remains a static, non-customizable, text-based medication tool,” added Catlin, who teaches and researches consumer behavior, health decision-making and marketing strategy/management, principles and regulatory issues.
The Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research opened a docket – FDA-2021-N-0353 – for potential changes in DFL requirements when it announced the workshop in April. The workshop, said OND Direct Theresa Michele, was a good launch toward changes for a rule the agency published in 1999.
“I was hoping we would get a few things we could take back and think about. We've gotten so much rich information from all of the speakers,” Michele said at the conclusion.
Along with Catlin’s presentation on research into consumer comprehension of DFLs, speakers during the day-long webinar discussed potential changes that could improve the labels’ efficacy in directing consumers’ self-selection of a nonprescription drug, as needed for some potential Rx-to-OTC switches.
 Some barriers to a dfl's effectiveness are beyond drug marketers' contro, JESSE CATLIN noted in slides aove and below as part of his PRESENTATION during FDA'S WEBINAR.
Source: Jesse Catlin/FDA webinar
Some barriers to a dfl's effectiveness are beyond drug marketers' contro, JESSE CATLIN noted in slides aove and below as part of his PRESENTATION during FDA'S WEBINAR.
Source: Jesse Catlin/FDA webinar
As FDA officials and industry stakeholders long have discussed, speakers in the webinar contemplated expanding the type of media allowed for DFL use from strictly text printed on paper or packaging to digital communications. (HBW Insight will publish additional coverage of the webinar presentations in future articles.)
Since publishing the DFL final rule in 1999, the FDA at multiple times has added to it or changed requirements for information, typically related to certain ingredients. But the DFL format or limits on how firms are allowed to present the required information have not changed. (Also see "NSAIDs Marketed In US Will Have Stronger Label Warning About Risks During Late Pregnancy" - HBW Insight, 15 Oct, 2020.)
“This is a great opportunity for us. I think we are on the cusp of a lot of neat things in the OTC marketplace,” Michele added.
Barriers Limit DFL Effectiveness
Catlin said his and others’ research has shown multiple barriers to a DFL’s communication of information, some of which are entirely out of control of a drug marketer or a regulatory agency (see images above).
One barrier, he said, is “the DFL struggles to communicate complex, multi-attribute criteria”
Consumers often struggle to understand and follow DFL information they must “simultaneously integrate multiple pieces or components of the information together, such as when a label requires a consumer to factor in medical history, age and other factors to make an accurate decision on whether to use a drug, Catlin said.
When considered as a decision tree, each point of a DFL has information or attribute a consumer needs to evaluate and reach an accurate self-selection decision.
“The DFL often performs quite well at any given individual node or component when viewed in isolation,” Catlin said.
“Where the DFL struggles is when we look at combined series of nodes, where the consumer has to factor in multiple items or criteria from DFL into their decision,” he added.
Studies of labeling for several OTC ingredients and comparing branded product DFLs with generics showed potential problems with stacking information in a DFL (see images below).
More Flexibility Would Help
Individual consumer characteristics also are a barrier to DFL efficacy. “Particularly those related to levels of ability, in terms of things like literacy, including health issues,” Catlin said.
And currently, the FDA’s DFL requirements allow drug marketers little room to make labeling more easily comprehended by a larger variety of consumers.
Source: Jesse Catlin/FDA webinar
“I would like to highlight the non-adaptive nature of DFL prohibits customization for individual needs. For example, space limitations prevent additional language options or different modes of communication for individuals with lower visual ability,” he said.
“The ability to customize label information could go a long way to helping these groups.”
Another consumer characteristic affecting DFL’s effectiveness is knowledge about a drug, or consumers’ belief in how much they know.
“Research shows that some consumers do not read the DFL at all or discard the packaging before use,” Catlin said.
“Obviously not reading the DFL is a problem as is discarding packaging for termination of use among various reasons could be an issue someone may be unaware of the development that would make them need to deselect or discontinue use or other users of the drug in the household may not have full information they consider using the drug.”
Beliefs Impede Information Uptake
Still, reading an entire DFL doesn’t ensure a consumer will follow all the instructions, beginning whether the drug is correct for a certain indication.
“Is also worth noting that even if the person reads the DFL, they may ignore or disregard the need to follow some or all of the instructions,” Catlin said, adding that research shows “some individuals believe they can choose their own dose regardless of what the package label says to do.”
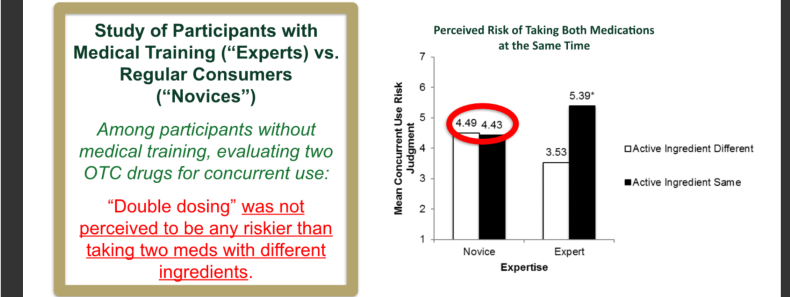
A similar barrier to a DFL’s effectiveness is a belief some consumers have expressed in studies that OTC drugs as relatively risk-free. “Even if used improperly such as by taking more than one medication with the same ingredient at the same time,” Catlin added.
Consumers also look to brands as labeling providing the information they need to pick a drug for a certain indication. With some brands expanding to include multiple indications, some with reaching indications and active ingredients that are different from a brand’s original product, the FDA has acted to curb firms’ use of what it calls “umbrella branding.” (Also see "US FDA: User-Error Risks Grow When OTC Brands Extend With Different Ingredients, Indications" - HBW Insight, 9 Dec, 2020.)
Relying on a brand as labeling is one of the “mental shortcuts” consumers use for choosing and using some OTC drugs, Catlin said. Not so with generic equivalents of OTC brands.
“We found less time spent reviewing the DFL and lower comprehension of the DFL when the principal display panel featured a brand instead of a generic. … You might think of branding as serving as a sort of mental shortcut where some individuals feel like they don't need to read the label as carefully if it is a more familiar brand,” he said.
Catlin recommended additional research of consumers’ reliance on branding as labeling. “I think it is worth thinking about how non-DFL elements of the packaging might interact with pre-existing individual characteristics to influence DFL communication.” (Also see "Eager For US Rule On Novel OTC Switches? Submit NDAs To Spur FDA Thinking – Gottlieb" - HBW Insight, 22 Mar, 2021.)
The FDA is looking at DFL label changes as it also modernizes and streamlines its OTC monograph drug program after Congress passed legislation in March 2020 authorizing the agency to make the changes. (Also see "‘OMUFA Fee Liable’ Trending In US OTC Drug Industry To Pay For FDA's Monograph Reform" - HBW Insight, 4 Jun, 2021.)
Also on its OTC drug plate and a recurring topic of discussion during the webinar are potential changes to its rules for OTC switch applications intended to expand the variety of drug ingredients available nonprescription by expanding drug marketers' options for labeling.




