US Consumers Don’t Know Where To Start With OTC Drug Fact Labels, Most Look Online For Help
Executive Summary
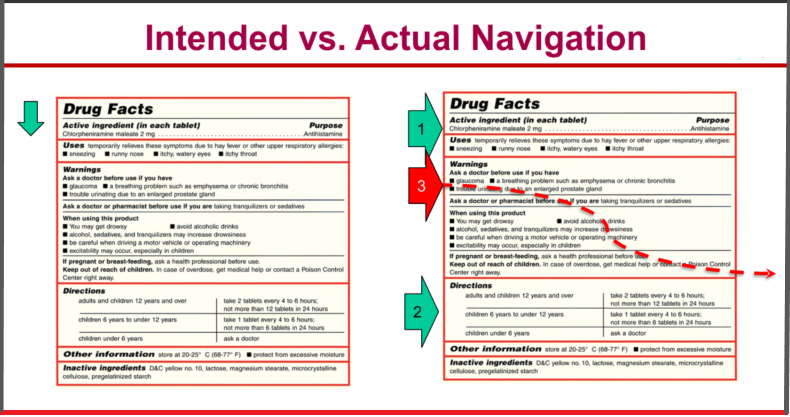
“We created a warning wallpaper, which means it looks like one block of overwhelming warnings,” says drug labeling expert Julie Akers. Technology has changed the world with nonstop access to information on multiple devices from myriad digital sources, but DFLs remain one-dimensional, static media.
US consumers are asked to understand a “wallpaper” of information on OTC Drug Facts labels, but most use little of it and rely more on what they find online, says drug labeling expert Julie Akers.
“The labels have gotten packed with more and more over the years and they are untenable for most consumers,” said Akers during a recent Food and Drug Administration online workshop on the strengths and weaknesses of DFLs and potential changes for the format required for nonprescription drugs by a 1999 final rule.
“The effects of the dense label are that one does not really quite know where to start,” added Akers, president and CEO of pharma industry consultancy Concentrics Research LLC.
That’s particularly apparent with consumers’ comprehension of warnings on DFLs. “We created a warning wallpaper, which means it looks like one block of overwhelming warnings,” she said.
Even when warnings text is read, some consumers don’t respond accordingly.
“We asked the question do you see these warnings as requirements or recommendations? Most consumers told us that they see these as recommendations,” Akers said.
The common reason behind those answers is most consumers, “especially those with chronic medical conditions, have a doctor, see a doctor regularly and they depend on what the doctor tells them over anything they agree read on the label,” she said.
Format Familiar, Ingredients Strange
Concentrics’ research also shows consumers struggle with DFLs even through they’re familiar with them. Ingredients, though, remain strange.
“Consumers will tell you that they're well aware there's a standard format and … know it has certain sections and they know it is on all the medications they currently are using,” Akers says
Additionally, consumers tell "us that while they are interested in the ingredients they can't pronounce them and don't really know what the active ingredient does or how it works."
“Some told us the warnings are for people who don't have a doctor and many don't believe they apply to them personally.”
Label Layers 'Invite Confusion'
Although the FDA currently limits DFLs to one-dimensional media – text and symbols printed on containers, packaging or inserts – some OTC labels add layers to the printed material.
That practice isn’t helping, consumers have told Concentrics during its 35 years’ of research, including more than 2m consumer interviews, on Rx and OTC drugs and devices.
“The expectations have grown to add more and more to labels. We now have peel-back and lift-off labels in order to pack it all in,” Akers said, adding, “We invite confusion.”
The DFL format the FDA requires is based on research and study data and other information available when the final rule was published. The agency at multiple times has added to it or changed requirements for information, typically related to certain ingredients, but the DFL format or limits on how firms are allowed to present the required information have not changed.
That’s the problem with DFLs, Akers observed. “Why is it not working? In short, the world changed.”
Technology has changed the world, opening nonstop access to information on multiple devices from myriad digital sources. But DFLs remain one-dimensional, static media.
“We have technology with everything we do now. It is in every part of our life and also fast, convenient, targeted and customized,” Akers said.
And societal shutdowns during the COVID-19 pandemic has only increased and accelerated consumers’ reliance on digital media for health care information.
“People are getting used to doing a quick Google search. That’s really what has changed and consider the pandemic only fueled the desire for technology and not only just the desire but absolute necessity for it,” Akers said.
“If I had to sum it up, why people use technology as an adjunctive the labeling I would say it is because it is searchable and customizable it is easy to do a search and you can learn specifically what you want to learn about and more portly it is fast and familiar device,” she added.
Package Front Important To Consumers
Adding extra-label digital components to OTC labeling is ahead in the US, but some improvements could help the current format.
The “principal display panel,” or front of package, is important to consumers, Akers said.
“Highlighting key conditions on the front of the panel is really helpful. Recently a participant told us they were seeking a cold medicine and found one that actually told them on the front they could use it if they have high blood pressure,” she said.
Particularly for low-literacy consumers, internet access, most often using smartphones, already is a common step in choosing an OTC remedy.
“It is already a DFL-plus technology world. This is where consumers review the label and use technology perhaps for searching the answer to a specific question. They do this because it is fast and easy,” Akers said.
 ADDITIONAL CONSIDERATIONs CONCENTRIC RESEARCH RECOMMENDS FOR CHANGES TO THE CURRENT DFL FORMAT. (SOURCE: CONCENTRICs RESEARCH/FDA WEBINAR)
ADDITIONAL CONSIDERATIONs CONCENTRIC RESEARCH RECOMMENDS FOR CHANGES TO THE CURRENT DFL FORMAT. (SOURCE: CONCENTRICs RESEARCH/FDA WEBINAR)
Navigating DFL Can Be Challenging
“Consumers want to understand what is involved in taking the medication first and they often skip to the directions, which means that the warnings are scanned,” Akers said.
“We find that consumers gravitate toward the purposes, uses and ask, what does a medicine do? They typically go to the directions section. They want to know how they use this medicine,” she added.
Potential side-effects also aren’t easily located on DFLs.
“It would be a huge improvement to simply retitle the heading that is called ‘when using this product’ to ‘side effects’ on labeling. Consumers tell us frequently that they wish they could find the side effects on the labels,” Akers said.
More space and less text also help, she added. “We see improved comprehension when our best practices included such things as white space, pictograms, special highlighting or bolding. Those treatments need to be used judiciously in order to have a desired effect.”
Dockets, Comments And Guidance |
|
The Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research opened a docket – FDA-2021-N-0353 – for potential changes in DFL requirements when it announced the workshop in April. Through 15 June, no comments had been submitted. The FDA also is encouraging drug firms to study making available nonprescription drug ingredients indicated for chronic conditions; in 2012 the agency opened a docket for recommendations on changing its OTC switch NDA rules to allow for “novel switches” targeting chronic conditions. Agency officials launched the initiative expecting labeling for novel switches to be subject to the same limits as other OTCs, with all necessary information available on a product’s label. However, the FDA has since indicated in a 2018 guidance that switch sponsors could tap into digital media and other extra-label tools to help demonstrate that consumers can assess whether an OTC drug is appropriate for them and they can use the product safely. (Also see "Before Asking FDA For Novel OTC Switch, Be Sure DFL Alone 'Can't Get There'" - HBW Insight, 23 May, 2019.) |