Florida Dental Products Firm Fails Regulatory Checkup With COVID-19 Claims
ACON Labs Recalls At-Home Tests Approved In Europe But Sold In US
Executive Summary
Products Iotech International sells to health care providers and consumers are deemed unapproved new drugs and are misbranded due to claims to prevent and treat COVID-19. ACON Laboratories determined Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing) is for sale in US without approval.
A Florida firm headed by a dentist and marketing mouthwash and hand sanitizers products with claims to prevent and treat COVID-19 is US regulators’ latest target concerning pandemic-related fraud in the consumer health sector.
Meanwhile, the manufacturer of OTC test kid for detecting COVID-19 available through an emergency use authorization reported that unauthorized, adulterated and misbranded versions of its product are being imported into the US.
 Iotech International was warned by fda and ftc about covid-19 claims for its iorinse mouthwash.
Source: Alamy
Iotech International was warned by fda and ftc about covid-19 claims for its iorinse mouthwash.
Source: Alamy
The Food and Drug Administration and the Federal Trade Commission in a joint warning letter published on 22 March alleged that products Iotech International LLC of Boca Raton, FL, was selling to health care providers and consumers from its website and social media pages, ioRinse mouthwash and ioCleanse Molecular iodine hand cleanser, are unapproved new drugs and are misbranded due to claims to prevent and treat COVID-19.
The agencies stated in the warning submitted on 15 March that no FDA-approved applications are in effect the products and they aren’t “aware of any adequate and well-controlled clinical studies in the published literature that support a determination that” the products are generally regarded as safe and effective e “for use under the conditions suggested, recommended, or prescribed in their labeling.”
Additionally, the active ingredients in the products are deemed Category III under an FDA OTC drug monograph and currently are allowed for use “as long as they meet the relevant conditions of use outlined in the applicable [tentative final monograph] comply with all other applicable requirements.”
However, COVID-19 claims aren’t covered in OTC monographs for oral rinses or topical antiseptics.
Iotech International’s”:labeling claims suggesting that your oral antiseptic rinse and topical antiseptic products are effective in inactivating and thus preventing infection or disease from the novel coronavirus that causes COVID-19 go beyond merely describing the general intended uses of an antiseptic as set forth” in the monographs for those products, the warning states.
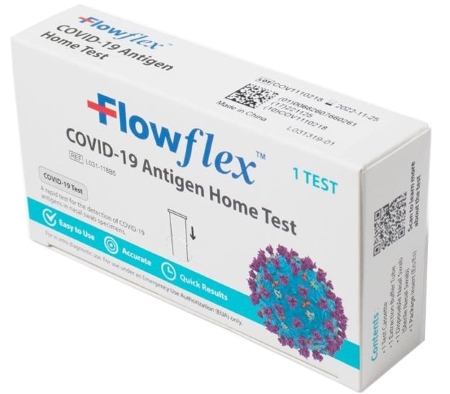
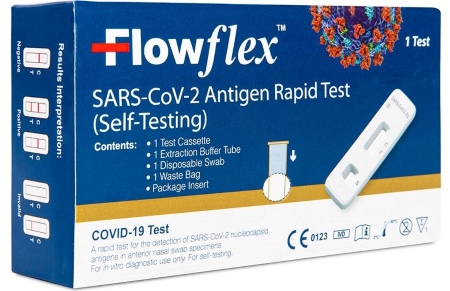 FLOWFLEX SARS-COV-2 ANTIGEN RAPID TEST (SELF-TESTING) kit, above, has approval for sales in europe but not the us and is on recall. Flowflex COVID-19 Antigen Home Test, below, is available in the US under an EUA.
Source: Alamy
FLOWFLEX SARS-COV-2 ANTIGEN RAPID TEST (SELF-TESTING) kit, above, has approval for sales in europe but not the us and is on recall. Flowflex COVID-19 Antigen Home Test, below, is available in the US under an EUA.
Source: Alamy
Moreover, the iodine in the firm’s hand cleanser isn’t one of the three active ingredients classified as Category III under the topical antiseptic TFM. The warning states that while “molecular iodine is not explicitly identified as an active ingredient on the label,” the label and labeling “clearly represent molecular iodine as an active ingredient, which is defined as a component of a drug intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease, or to affect the structure or function of the body “
Like the other firms the FDA has warned about making bogus COVID-19 claims, whether for dietary supplements or for drugs, the agency instructed Iotech International to respond within 48 hours, rather than the 15 days allowed following other warnings, with the specific steps taken to correct violations.
The FDA’s list numbers 206 through 22 March. Between its own warnings and its joint letters with the FDA, the FTC has submitted warnings to more than 400 businesses about COVID-19-realted consumer product fraud.
CE There, EUA Here
San Diego-based ACON Laboratories Inc. in January, according to an announcement the FDA posted on 11 March, determined that counterfeit copies of its Flowflex COVID-19 Antigen Home Test were being sold in the US under the brand Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing).
The US office of ACON Biotech (Hangzhou) Co., Ltd. in China stated it isn’t importing Flowflex SARS-CoV-2 (Self-Testing) into the US as the product, made by the parent firm, is authorized in the European Union under a CE mark, which allows the product’s sale in all EU markets and indicates conformity with member states’ mutual standards.
Flowflex COVID-19 Antigen Home Test, which is available in the US under an EUA, cannot be legally imported, distributed or used in the European market as it is not CE-marked.
The firm’s announcement follows a consumer warning the FDA published on 1 March against using Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing) because it had not been authorized, cleared, or approved for distribution in the US. (Also see "FDA Warns Against Use Of 3 Unapproved COVID-19 Tests" - Medtech Insight, 1 Mar, 2022.)
The firm’s recall announcement also noted that COVID-19 antigen tests sold in the US without FDA approval, clearance or authorization, or in Europe without a CE mark, pose significant risk as they may lead to false negative or positive test results. However, it had not received any reports of adverse events related to the products when the announcement was published.