OTC Monograph User Fees Grow In FDA FY2023 Budget Request And In Industry's Thinking
Executive Summary
Stakeholders didn’t indicate extensive familiarity with OTC monograph user fees during online workshop. Biden administration’s FDA FY2023 budget request includes $5m increase to $30.36m in facility registration fees required from all firms manufacturing OTC monograph drugs available in US.
User fees for the Food and Drug Administration’s OTC monograph program will be part of the agency’s third consecutive budget with its fiscal year 2023 appropriation, but the fees and the operations of the program they support still are new to some in the industry.
The OTC monograph program overhauled to be more effective and efficient under 2020 legislation resembles the FDA’s process for prescription drug applications for adding ingredients and indications and for scheduling meetings application sponsors will have with agency officials.
Potential users of the program, though, didn’t indicate extensive familiarity with OTC monograph user fees or the program’s application and meeting process during an online workshop the Office of New Drugs Division of Nonprescription Drugs in the agency’s Center for Drug Evaluation and Research conducted on 29 March.
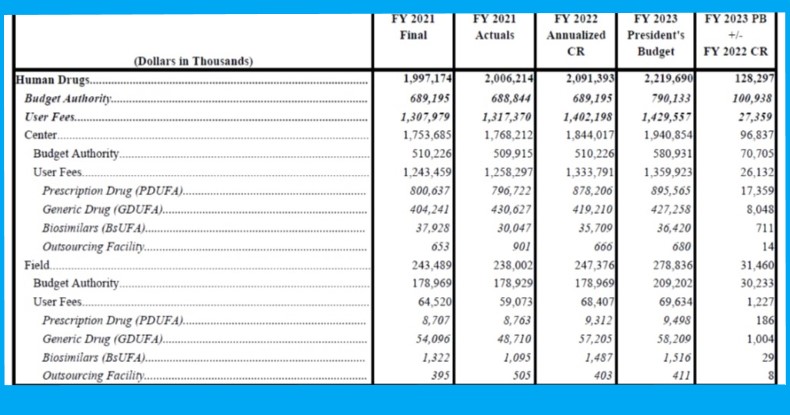
The Biden administration’s request for the FDA’s FY2023 budget includes an increase of slightly more than $5m to $30.36m in annual facility registration fees which are required from all firms manufacturing OTC monograph drugs available in the US (see table above).
The user fee program also imposes fees on sponsors, or requesters, of OTC monograph order requests – OMORS: $500,000 for Tier 1 requests to develop monographs for new ingredients or add new medical conditions to existing monographs, and $100,000 for Tier 2 OMORs for modifications to drug facts labels, standardization of the concentration or dose of a finalized ingredient within a finalized monograph, a change to ingredient nomenclature or other similar changes. (Also see "US FDA Meets Initial Deadlines In OTC Monograph Overhaul While It's Rebuilding The Program" - HBW Insight, 5 Oct, 2021.)
The total budget the White House requests for the FDA in FY2023 is $8.4bn, a nearly 34% increase over its FY2022 appropriation; $2.22bn of the total would go to the agency's human drug programs, with $790m in direct appropriation and $14.3b in user fees (see table below).
‘What Are These Fees?’
The OTC monograph workshop, covering information from a draft guidance published in February on scheduling meetings with agency officials to discuss OMORs, prompted questions to FDA officials which signaled some lack of knowledge about the overhauled monograph program. (Also see "'Milestone' Discussions With US FDA On OTC Monograph Proposals Limited To One Meeting" - HBW Insight, 23 Feb, 2022.)
“You said that a meeting request could be denied for failing to pay your user fees. What are these fees?” one participant asked.
However, although the consequences of failing to pay the fees – OMUFAs – was part of the presentations by Trang Tran, a senior regulatory health manager in the Division of Nonprescription Drugs, and Elizabeth Thompson, project management staff in the division, defining the fees wasn’t part of the draft guidance and wasn’t part of the webinar.
 FDA'S FY2023 drug PROGRAMS FUNDING WOULD BE AN INCREASE OF MORE THAN $128M FROM ITS CURRENT APPROPRIATION AND WOULD BE SPLIT BETWEEN $1.43bn for CDER $278.3M FOR RELATED WORK BY OFFICE OF REGULATORY AFFAIRS.
Source: FDA
FDA'S FY2023 drug PROGRAMS FUNDING WOULD BE AN INCREASE OF MORE THAN $128M FROM ITS CURRENT APPROPRIATION AND WOULD BE SPLIT BETWEEN $1.43bn for CDER $278.3M FOR RELATED WORK BY OFFICE OF REGULATORY AFFAIRS.
Source: FDA
Tran and Thompson, both assigned to the FDA as commanders in the US Public Health Service, fielded questions about failing to pay user fees after covering the topic in their presentations. The potential for one member of a joint OMOR, which the FDA is calling OTC industry working groups, to stall a proposal by being in arrears in user fees was one of those questions.
“As noted in the presentation, all members of the OTC IWG and their affiliates who are subject to fees must not have any unpaid user fees in order to participate in an over-the-counter monograph meeting,” Thompson said.
FDA will close the door even after scheduling meetings. “FDA will deny a meeting request or cancel a previously scheduled meeting if the meeting requester and or its affiliates who are subject to fees, have any unpaid user fees,” she said.
Timeframe Clock Not Ticking Yet
When the FDA will begin scheduling OMOR meetings also is generating questions. Some probably are because the agency’s accepting requests, but under an agreement with industry included in the legislation authorizing monograph program overhaul, it’s not on the clock yet to conduct meetings.
“FDA has been reviewing and acknowledging meeting requests in the order of receipt. However … applicable meeting timelines and performance goals agreed to by FDA and industry in the proof of commitment letter are not effective until October 1 of this year,” Tran said.
Asked whether the FDA, for meeting requests submitted after the draft guidance was published, would respond within the timeframes stated in the document, Tran also referenced that meeting request response time goals and other management performance goals are not effective until 1 October.
“Therefore, meeting requests submitted before this date will not be processed under specified timeframes outlined in the OMUFA commitment,” she said.
Those timefames are 14 days for Type X meetings, necessary for an otherwise stalled OTC monograph order development program to proceed or for important safety issue that needs immediate action, and for Type Y meetings for overall data recommendations and pre-OMOR submission.
For Type Z meetings, defined as “any meeting that is not a Type X or Type Y meeting, the timeframe for an FDA response is 21 days.
Interest from the drug industry in using a more effective and efficient OTC monograph process has been growing since long before the agency was authorized to overhaul the program. The FDA’s representatives on the webinar were careful to temper stakeholders’ expectations for when to expect to start using the process.
Asked when the FDA will begin scheduling OMOR meetings, Thompson also noted that the agency’s response time goals aren’t effective until 1 October.
“And FDA only needs to meet 50% of the total of meeting management goals for that fiscal year, which includes meeting response, scheduling, preliminary responses and meeting minutes. After that, the goals for these meetings will increase over time to allow FDA adequate time to ramp up the program,” she said.
