US FDA Testing ‘Discount’ Multivitamins 'Very Soon’
Executive Summary
“Over the last year or so, we've had some concerns about the quality of some of the discount multivitamins that are on the market,” says Michael Dutcher, director of the ORA Office of Human and Animal Food Operations' Minneapolis region.
Manufacturers and marketers of “discount” multivitamins could face questions from the US Food and Drug Administration about the products’ labels and formulations, says an official in the agency’s inspections office.
“Over the last year or so, we've had some concerns about the quality of some of the discount multivitamins that are on the market,” said Michael Dutcher, deputy director of the ORA’s Office of Human and Animal Food Operations at the Dietary Supplement Regulations Summit on 21 July in Washington.
Dutcher said ORA will “be issuing an assignment … very soon” focused on “the accuracy of nutrient content claims on the labels of discount multivitamins.”
Speaking via an online link during the conference conducted in-person and virtually, he didn’t elaborate on which multivitamin brands or lines the FDA sees as discount products.
Most retailers and other distributors selling multivitamins and other dietary supplements offer national brand products and lower-priced private label and store brand versions.
Dutcher, based in Minneapolis in ORA's region including Minnesota, North Dakota, South Dakota and Wisconsin, explained that inspectors’ concerns may not be borne out in the planned testing and surveillance.
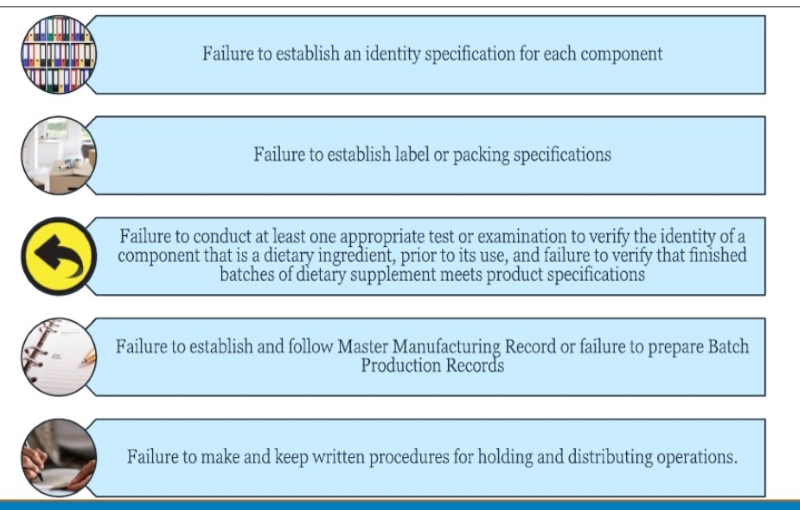
US FDA Top 10 Dietary Supplement Inspection Findings
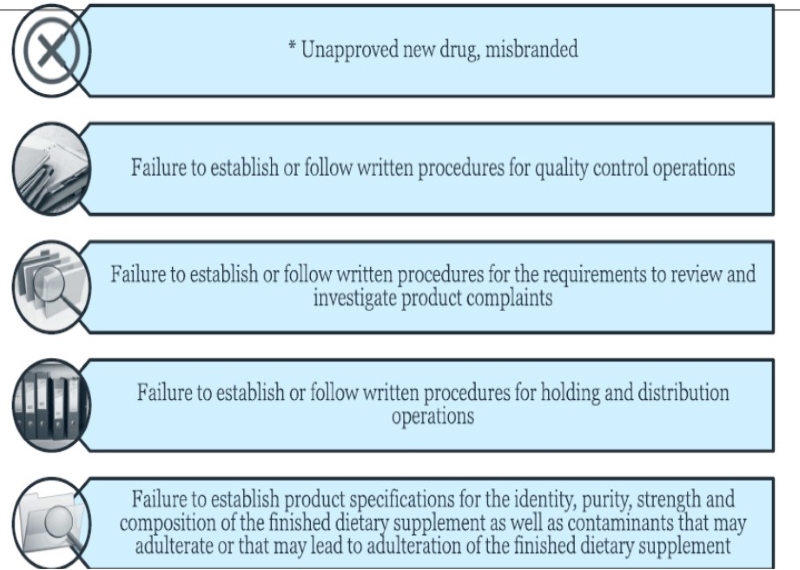 Potential non-compliant Labeling for some multivitamins, which is a concern FDA officials have noted, is among the most commong problems, above and below, the agency finds in supplement facility inspections.
Source: FDA
Potential non-compliant Labeling for some multivitamins, which is a concern FDA officials have noted, is among the most commong problems, above and below, the agency finds in supplement facility inspections.
Source: FDA
“The goal here is to test them and make sure we're not experiencing any issues with multivitamin that are either super-potent, or under-potent, or may have contaminants in them that we're unaware of,” he said.
“It may not even be an issue, but we don't know without going out and doing some surveillance sampling to find out this assignment will give us an indication as to how manufacturers are formulating their discount dietary multivitamins.”
Solutions to any problems, he added, would come with industry input. "If we find that there's an issue there, then it's something we'll just have to work within industry on to try and correct.”
Labeling Among Top 10 Problems
Dutcher also noted that ORA officials' concerns about some multivitamins dovetail with common problems they’re finding at supplement facilities during inspections for compliance with the 2007 final rule for supplement good manufacturing practices. (Also see "Basic Supplement GMP Compliance Problems Continue In FDA Warnings" - HBW Insight, 8 Feb, 2017.)
He said the 10 most common problems are led by failing to comply with labeling requirements, although those findings aren’t noted in inspection reports.
“We generally don't cite labeling, labeling issues as an observed action report, so it won’t turn up on inspection reports. But it is one of the more common issues that we identified during our inspections.”
Non-compliant labeling often is claims for drug or disease indications, which render a supplement an unapproved drug which is misbranded because it’s not labeled with directions appropriate for the indication. (Also see "Warnings Note Disease Treatment Studies Placed Too Close To Supplements For US FDA’s Comfort" - HBW Insight, 22 Jan, 2021.)
“I'm sure for folks in the room today, that's not really anything new,” Dutcher acknowledged.