VCRP Product Reporting To FDA Drops 25% In FY 2014
This article was originally published in The Rose Sheet
Executive Summary
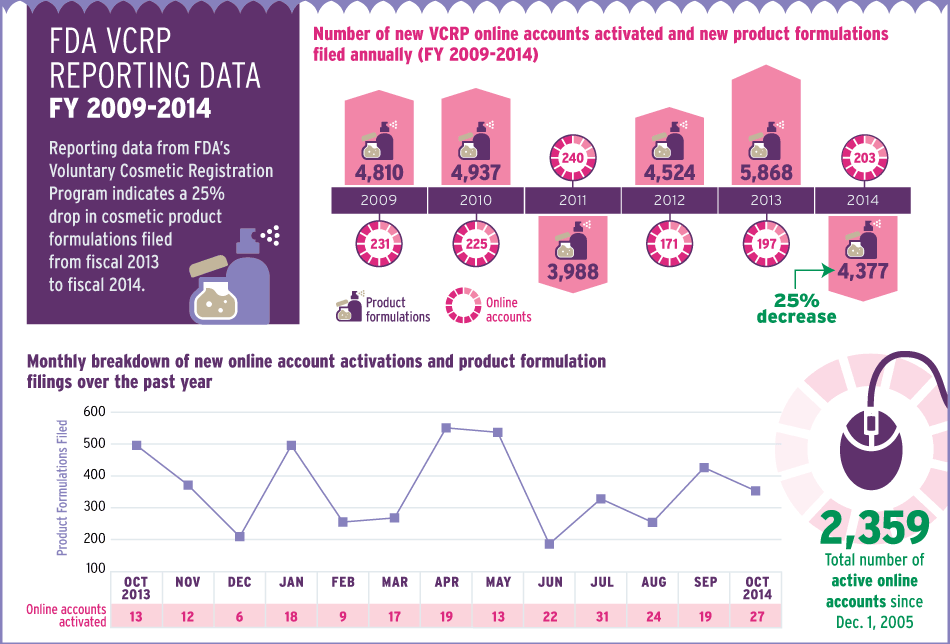
The number of cosmetic product formulations reported to FDA through its Voluntary Cosmetic Registration Program dipped 25% from fiscal 2013 to fiscal 2014. A detailed infographic tracks participation in the program over the past six years.
Cosmetic product formulations reported to FDA in fiscal 2014 under the Voluntary Cosmetic Registration Program totaled 4,377, a 25% drop from the prior-year total of 5,868, according to data obtained through a Freedom of Information Act request by “The Rose Sheet.”
Fiscal 2014’s product formulation reporting was the second-lowest reporting year over the past six years. Only 2011 was lower with 3,988 formulations reported.
The 2014 drop could partially be attributed to companies culling product offerings as they focus on streamlining their portfolios and offering fewer, bigger-impact items to consumers. Firms including Procter & Gamble Co., [Beiersdorf Inc.] and [Avon Products Inc.] have all announced plans to pare down their cosmetic and personal-care product lineups to cut costs and ensure that big innovations stand out.
In an email to “The Rose Sheet,” an FDA spokeswoman said the agency could not comment on product-formulation reporting increases or decreases because participation in the program is voluntary.
According to FDA’s website, information gleaned from VCRP reporting helps the agency evaluate cosmetic products on the market.
“Because product filings and establishment registrations are not mandatory, voluntary submissions provide FDA with the best information available about cosmetic products and ingredients, their frequency of use and businesses engaged in their manufacture and distribution,” FDA says.
In addition, information submitted through the VCRP assists the Cosmetic Ingredient Review Expert Panel in selecting ingredient review priorities and assessing cosmetic ingredient safety.
During a 2012 Capitol Hill hearing on cosmetics issues, Michael Landa, director of FDA’s Center for Food Safety and Applied Nutrition, suggested that VCRP figures at that time represented only one-third of cosmetics manufacturers (Also see "House Hearing Probes FDA’s Cosmetics Role" - HBW Insight, 2 Apr, 2012.).
As momentum builds to overhaul FDA’s oversight of the cosmetics industry, stakeholders and legislators have proposed making aspects of the VCRP mandatory.
Introduced in 2012, the Cosmetic Safety Amendments Act would have instituted registration requirements and mandated that firms submit product statements to FDA listing ingredients used (Also see "Council-Approved Cosmetics Safety Bill Launches" - HBW Insight, 23 Apr, 2012.). The bill had the full support of industry trade group the Personal Care Products Council.
In the absence of a regulatory mandate, PCPC urges its member companies to participate in the VCRP as part of its Consumer Commitment Code.