CBD Market Survival-Of-Fittest Looms With FDA Regulation, Industry Standards
Executive Summary
Number of hemp and CBD supplement marketers will shrink as passage of farm bill leads to industry certification as well as FDA enforcement. Firms practicing transparency are most likely to survive in a few years.
Industry-supported standards and growing retailer participation on top of looming FDA regulatory enforcement will bring order to the hemp and cannabidiol dietary supplement market currently marked by chaos amid booming sales, say executives with two firms.
Likening the rush of firms competing in the field to the explosion of alcoholic beverage marketers in the US following the end of prohibition in 1933, they say FDA's initiative to establish regulatory pathways for using hemp and CBD ingredients in supplements and food, prompted by the December re-authorization of US Department of Agriculture programs that lifts some restraints on hemp research, production and sales, will be key installing standards for safety and quality in the market.
Hemp is one of more than 80 active chemicals in marijuana and is defined in the farm bill as having less than 0.3 percent concentrations, on a dry weight basis, of THC, the psychoactive compound in the plant. Like CBD, hemp does not cause intoxication or euphoria that is caused by THC.
US Hemp-based Food Sales
Hemp-based food sales in the us have increased steadily and strongly since 2012 and are expected to continue growing at similar rates,
New Frontier Data report from Hemp Business Journal
Chris Husong – VP of marketing and communications for Broomfield, Colo.-based firm Elixinol LLC – says firms that focus on quality and the overall good of the entire category will survive an inevitable paring down of the space.
“There will be some products and brands that will be cannibalized or merged into others, but primarily the brands that are doing it right, the brands that have the right story, the brands that are vertically integrated, the brands that are working not only to be transparent and create great quality products but that are also working with different industry groups to make sure all the standards are set are the ones that will still be around,” he said in an interview.
Annie Rouse, co-founder of Anavii Market LLC – a Lexington, Ky.-based online retailer of CBD brands – also made the comparison to alcohol, predicting the field will shape up over time to look much like the beer market. “You will have your massive players, like Coors, Budweiser and Miller and then you have your small microbrews which are very localized,” Rouse said an interview.
She said “way too many” firms currently are in the market. “But I think in general that will end up shaking up over time and we still haven’t even penetrated more than 5% of the US market, so I think there will be plenty of space for industry to blossom.”
Currently, hundreds of US firms market hemp and CBD supplement and personal care products in numerous categories, from CBD tinctures and oils to extract supplements, teas and topical lotions. Products are positioned to address inflammation, pain and mood.
Market research firm IRI estimates CBD-containing consumer health and personal care product sales in the US already have reached $500m. "I have heard some higher figures than that," said global consumer health business consultant Nicholas Hall at a recent industry conference (see sidebar below).

"In time, my view is that OTC CBD, registered and regulated with claims, will come. It won't come first in the United States, but I think in the next five years we'll have it in countries like New Zealand the UK and other places. It will be big," Hall said. Separately, US hemp-based food sales alone were $124m in 2018 and are expected to reach $137m this year and $186m by 2020 (see chart above).
Big Chains Eyeing Field
Industry insiders have predicted the market will see a big surge after Congress passed the farm bill with a provision allowing production and marketing of hemp food, supplement and personal care products while also expanding the amount of research allowed on the plant. (Also see "Farm Bill Hemp Provision Lights Another Supplement Industry Regulatory Priority" - HBW Insight, 25 Jan, 2019.)
Husong noted 170 CBD and hemp product marketers attended the Expo West trade show in Anaheim, Calif., earlier in March, and the show buzzed with excitement over the products. “Every national retailer wanted to talk” about the category, he said. As far as whether big chains will carry CBD- and hemp-products, he said “its’ not a matter of if, it’s when.”
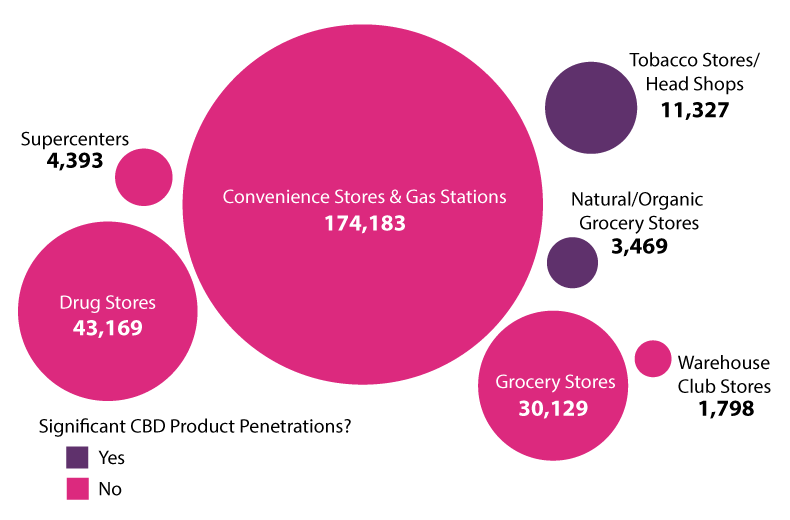
Convenience stores and gas stations, which number 174,183 across the US, account for the majority of hemp and CBD products sales, followed by drug stores and grocery stores (see chart below).
CVS Health Corp. is rolling out hemp-derived CBD products in some stores in California, Colorado, Illinois, Kentucky, Maryland and Tennessee, said Michael DeAngelis, the firm's senior director for corporate communications. The products include creams, sprays, roll-ons, lotions and slaves, which are marketed under third-party brands.
“We are working with CBD product manufacturers that are complying with applicable laws and that meet CVS’ high standards for quality,” DeAngelis said in an email. Asked why CVS is moving into the category in a bigger way, he said, “CBD is gaining popularity among consumers, particularly those looking for alternative care products.”

FDA currently does not prevent marketing of hemp- or CBD-containing supplements that are compliant with supplement labeling and manufacturing requirements, particularly if the products are not labeled or promoted with drug claims. The practice is a deviation from its regulations, which prohibit using in supplement ingredients contained in approved drug products or that are being studies in pharmaceutical tests – CBD is an active ingredient in an FDA approved drug and it and tetrahydrocannabinol, the psychoactive compound in cannabis, are subjects of clinical investigations.
The farm bill provision prompted FDA to consider changes that would allow use of cannabis ingredients in dietary supplements. A working group will lead FDA's decisions for allowing the ingredients in nutritional products following a yet-to-be-scheduled public meeting for comment on the agency's plans. (Also see "House Appropriators Stoke Flames For FDA Regulation On CBD In Supplements" - HBW Insight, 1 Mar, 2019.)
The Natural Products Association has criticized FDA’s delay in creating an enforcement strategy for products, arguing the lack of action allows bad players to enter the market and avoid scrutiny. (Also see "CBD Market 'Surges' As FDA Delays Setting Regulatory Policy – NPA" - HBW Insight, 21 Mar, 2019.)
Transparency: Bedrock Of Survival
Husong said transparency is an important trait for any brand to survive over the next few years. “You have to make sure that the product that is grown is as pure and organic as you want your end product to be,” he said. “Where your source your product is one of the most important things. Next is how you label the product, how it’s made, its origins.”
Elixinol provides country of origin for the 38 SKUs for its namesake brand in four product categories: CBD capsules, hemp-CBD oil tinctures and oil liposomes and water-soluble CBD powder. The formulas are high-quality and focus on bioavailable delivery systems, Husong said.
The firm is a member of the US Hemp Roundtable, which has worked with The Hemp Authority to develop the first certification seal for hemp-containing products.
On March 20, the roundtable announced it first certification seal awards, to 13 unidentified companies. The companies that meet “stringent” standards, including requirements on processing and production, supplier qualification, good manufacturing practices and record-keeping, including records of country of origin. Firms that pass a third-party audit can carry the seal, according to the Hemp Authority’s website.
The roundtable in 2018 launched its guidance plan 1.0, its first attempt to “demonstrate the industry’s professionalism and to promote confidence among consumers that hemp products are safe and among government officials that hemp products meet legal standards.” The association is working on guidance 2.0 to improve its strategy.
Husong said Elixinol and the roundtable continue to work on product labels, which are a challenge for the industry. “A lot of what has been established now came from the marijuana world and we’re moving into the dietary supplement and health and wellness world. Instead of talking about percentage and dosages, we’re going to be talking about serving size and price per serving and how that all works,” he said.
