Magnesium Qualified Health Claims For Reducing Risk Of Hypertension Authorized In US
Executive Summary
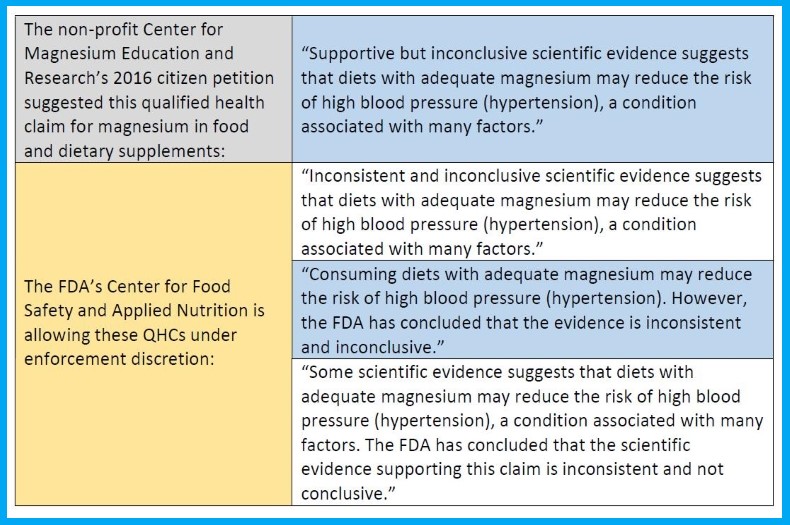
CFSAN decides on Center for Magnesium Education and Research LLC's 2016 petition requesting FDA authorize a qualified health claim about relationship between magnesium and reduced risk of high blood pressure, or hypertension.
Dietary supplements marketed in the US with indication for daily dosage of magnesium up to 350 mg can begin using qualified health claims for reduced risk of high blood pressure under Food and Drug Administration enforcement discretion.
The agency’s Center for Food Safety and Applied Nutrition on 10 January announced its decision on a citizen petition submitted in 2016 by the Center for Magnesium Education and Research LLC – docket FDA-2016-Q-3770-0001 – requesting that the FDA authorize a qualified health claim (QHC) about the relationship between magnesium consumption and a reduced risk of high blood pressure, or hypertension.
Based on the data and information it considered, the FDA concluded that the use of magnesium in supplements at levels necessary to justify the QHCs, which are at the ingredient’s daily upper level, is safe. Single-ingredient and other magnesium supplements and food products containing a sufficient amount of it among other ingredients and conventional food products will be allowed to use the claim.
Claudine Kavanaugh, CFSAN Office of Nutrition and Food Labeling direction, said in a letter of enforcement discretion to the non-profit Center for Magnesium Education and Research (CMER) that its petition “demonstrated to FDA’s satisfaction that magnesium in conventional foods and dietary supplements, as long as it does not exceed 350 mg/day in dietary supplements when used according to their labeling, to be safe and lawful.”
However, CFSAN rejected the QHC that nutrition science and regulation expert Guy Johnson suggested in the petition he filed for the CMER. The agency instead stated three options for use as a QHC for reduced risk of hypertension on qualifying magnesium supplements – two of the options are similar to the petition’s suggestion in length and detail, while the third is much longer and detailed (see table below).
Two Similar, The Third Not So Much
QHC Targeted In Petition
The CMER isn’t surprised the FDA didn’t authorize the suggested QHC and it’s continuing research to support petitioning the agency for additional QHCs for magnesium.
“We were sort of expecting it. We would have liked to them to go a little bit further, but we understand it. The reason we understand that is because there's a tremendous amount of studies on magnesium, and hypertension, and magnesium and blood pressure,” said CMER direct Andrea Rosanoff.
The FDA is accurate noting inconsistencies in research on magnesium’s health benefits, Rosanoff said in an interview. Eliminating inconsistencies in research and offering results that the FDA finds conclusive eventually could lead to an unqualified health claim for magnesium and reduced risk of hypertension.
“They said there's a lot of inconsistency in the results, and that we have known for a long time,” she said.
Third QHC In Three Years
The magnesium QHC is the first one the FDA has authorized for supplements and food since July 2020 when it rejected a health claim for supplements requested in a petition Ocean Spray Cranberries Inc. submitted regarding the relationship between the consumption of cranberry products, used in food and beverages as well as supplements, and the reduced risk of recurrent urinary tract infection in healthy women. (Also see "US FDA Again Stops At Qualified Health Claim For Supplements: Cranberry Reduces UTI Risk" - HBW Insight, 21 Jul, 2020.)
Instead, the CFSAN said it would exercise enforcement discretion regarding claims about the association between a reduced risk of recurrent UTI and the use of supplements containing at least 500 mg cranberry 100% fruit powder and of cranberry juice beverages containing at least 27% cranberry juice, an amount most commercially available cranberry cocktails contain. N MMM
The decision on cranberry QHCs came a year after the FDA announced enforcement discretion for five additional QHCs for blood pressure and coronary heart disease risk reduction to be used with omega-3 supplements. (Also see "FDA Holds Line On Omega-3 Claims, But Gives Enforcement Discretion For More" - HBW Insight, 19 Jun, 2019.)
Those QHCs were added in response to a petition by the Global Organization for EPA and DHA Omega-3 seeking unqualified health claims for eicosapentaenoic and docosahexaenoic fatty acids in food or supplements.
Supplement industry stakeholders noted the agency’s decision on EPA and DHA QHCs showed trepidation at the FDA about allowing claims that approach resembling indications for approved drugs with botanical-based active ingredients. (Also see "Omega-3 Heart Health Claims Decision: FDA Out Of Step With Industry?" - HBW Insight, 1 Jul, 2019.)
“We are in the process now of doing a statistical meta-analysis on what we see as the reason for the inconsistencies. … With the work that we're doing now we're going to be able to provide [the FDA] with the high standards that they require for a health claim.”
Johnson, principal of Johnson Nutrition Solutions LLC in Minneapolis, told HBW Insight that health claim petitions for dietary and food ingredients typically ask for unqualified claims only to be either rejected outright or authorized for QHCs instead.
“We asked for a qualified health claim petition, we felt that it would be, well, to be brutally honest, FDA is only added three or four on qualified health claims since health claims came into existence in 1993,” he said.
Health claims must be the significant scientific agreement standard the FDA recognizes. “significant scientific agreement is a very, very high standard,” Johnson said.
FDA authorization of QHCs through enforcement discretion is its only option for allowing use of the claims other than rejecting requests made in petitions.
“Technically, what they're doing is saying, we have not authorized an unauthorized health claim, but we will, we are comfortable with you making the claim that you've outlined under these conditions using as long as you include the language of that health claim of the qualified health claim in your materials,” Johnson said.
Notably, ending enforcement discretion for a QHC, or for any other purpose the FDA exercises enforcement discretion, can be done without a public rulemaking process, though the agency hasn’t reversed course on any QHC so far.
“It's not codified in the Code of Federal Regulations. But it' the mechanism they use to tell you what they're comfortable with and what they're not,” Johnson said.
At Least 20% Of Magnesium DV
Johnson noted in the petition that the claim would apply to conventional foods and supplements that contain at least 20% of the Daily Value (DV) of magnesium per reference amount customarily consumed (RACC).
For nutrition labeling purposes, the DV is 400 mg per day. That level would qualify products as “high” sources of magnesium as defined in agency rules.
The petition also proposed that foods and supplements using the claim would comply with all of FDA general requirements for health claims; tree nuts, which are high in magnesium, would be exempt from a total fat disqualifying level.
Kavanaugh pointed out in the letter to the CMER that data from the 2015-2016 National Health and Nutrition Examination Survey indicated the mean daily magnesium intake from foods and beverages, excluding supplements, was 289 mg for all persons 2 and over.
For consumers 19 and up, the Recommended Dietary Allowance for magnesium intake is 400-420 mg/day for men and 310-320 mg/day for women. The DV for magnesium is 420 mg for adults and children 4 and older, 400 mg for pregnant and lactating women and 80 mg for children 1 to 3 years old.
Based on those levels, the “FDA believes that the proposed qualified health claim would be unlikely to result in a mean dietary intake of magnesium from foods and beverages that would exceed the DV of 420 mg for adults and children greater than 4 years of age,” Kavanaugh wrote.
For food, the CFSAN says a product must meet the agency’s “low sodium” standard – containing less than 140 mg sodium per RACC – to use a magnesium and hypertension QHC.
‘Consistency Of Findings Important’
She also explained that based on the findings from 38 intervention studies deemed relevant, FDA concluded “some credible evidence” suggests a relationship between the intake of elemental magnesium from foods and supplements and reduced risk of hypertension.
“However, this evidence is inconclusive because the results of the studies are inconsistent,” Kavanaugh stated.
“Consistency of the findings among similar and different study designs is important for evaluating causation and the strength of the evidence. Lack of consistency among studies evaluating the same substance-disease relationship weakens the strength of the evidence,” she added.
Findings in the minority of intervention studies showing a statistically significant lowering of blood pressure from intake of magnesium are undermined by the larger body of evidence of no effect on blood pressure. In studies conducted in subjects with hypertension, results were inconsistent in subjects with normal or elevated blood pressure in both diastolic and systolic measures.
Results also were inconsistent in studies conducted in subjects with pre-existing cardiovascular diseases, including coronary artery and other forms of coronary heart disease.
Additionally, results were inconsistent across all levels of magnesium intakes and ingredient formats – from 181 mg to 960 g daily; study participants’ ages – mean ages 20 to 71; study durations – two weeks to six months; and sample sizes – 7 to 227 subjects using magnesium.
The Council for Responsible Nutrition, the only supplement industry trade group among the organizations and businesses sponsoring CMNER's work on the petition, says the FDA is giving magnesium the nutrition credit it deserves.
“We are pleased FDA recognizes the role of magnesium in reducing the risk of hypertension in addition to this essential nutrient’s many other functions in the body. CRN’s contribution to the petition is an example of our continued commitment to scientific research to advance regulatory and nutrition policy change,” said Andrea Wong, CRN’s senior vice president of scientific and regulatory affairs, in a statement to HBW Insight.