Outlook 2024
Annual Industry Ranking And Forecast
VIEW NOWHBW Insight is part of Pharma Intelligence UK Limited
This site is operated by Pharma Intelligence UK Limited, a company registered in England and Wales with company number 13787459 whose registered office is 5 Howick Place, London SW1P 1WG. The Pharma Intelligence group is owned by Caerus Topco S.à r.l. and all copyright resides with the group.
This copy is for your personal, non-commercial use. For high-quality copies or electronic reprints for distribution to colleagues or customers, please call +44 (0) 20 3377 3183
Printed By



Views from industry leaders and experts on the biggest trends, challenges and opportunities facing consumer health in the new year.

Walmart Inc. sent a brief to all its beauty and personal-care vendors detailing expectations for brands and other partners under the Modernization of Cosmetics Regulation Act, noting violations of the law breach its supplier agreements.

The Personal Care Products Council seeks to stem the rising tide of titanium dioxide Proposition 65 lawsuits, requesting that a California court prohibit the state’s Attorney General and private enforcers from filing and/or prosecuting new suits against cosmetics companies failing to warn about potential TiO2 exposure.

CRN’s request for clarification, as it continues litigating complaint in US District Court for Southern New York, highlights what it contends is vague and overly general language in the legislation passed in October with a 22 April effective date.

CA, MA and NJ legislatures remain in session with bills active proposing regulations similar to NY law effective 22 April requiring retailers, including online, to ask for proof of age when customers buying consumer health products containing ingredients labeled or promoted for weight loss and bodybuilding benefits appear younger than 18.

CRN’s request for clarification, as it continues litigating complaint in US District Court for Southern New York, highlights what it contends is vague and overly general language in the legislation passed in October with a 22 April effective date.
Firm operating in London, India and United Arab Emirates says its “Ultra Factory” will open in Indiana within the next six months with end-to-end production based on its operational facility in India.

CA, MA and NJ legislatures remain in session with bills active proposing regulations similar to NY law effective 22 April requiring retailers, including online, to ask for proof of age when customers buying consumer health products containing ingredients labeled or promoted for weight loss and bodybuilding benefits appear younger than 18.

Federal judge finds “misreading of the legislation” in CRN’s argument that state “restricts access based purely on what has been said about the product or its ingredients.” But standing to challenge “means that only CRN is positioned right now to go before the court on behalf of industry,” says CEO Steve Mister.

Companies considered producers of single-use packaging in Oregon, Colorado and California must register with Circular Action Alliance, the leading (and currently only) producer responsibility organization, by 1 July 2024 under new state recycling laws.
There’s no simple answer to the question of how to make medicines packaging sustainable, says international recycling consultancy and Veolia Group subsidiary Circpack. “Manufacturers need to assess what works best for them, and recyclers will do the same,” Circpack analyst and circular packaging expert Filipe Vieira de Castro tells HBW Insight in this exclusive Q&A. “The best thing we can all do is put effort into finding common ground between systems, which comes down to better understanding the complexities between them.”
The EU Parliament's Environment, Public Health and Food Safety committee proposes a “compromise amendment” to the new pharmaceutical directive that would include only antimicrobials for which there is an “identified risk of antimicrobial resistance” to the prescription requirement.
The EU Parliament's Environment, Public Health and Food Safety committee takes a compromise position with regards to the Packaging and Packaging Waste Directive. Medicines and medical devices should be exempt, but only until 2035, at which point the European Commission should check whether the development of materials and the recycling process have progressed, and may adjust this exemption accordingly.

CRN’s request for clarification, as it continues litigating complaint in US District Court for Southern New York, highlights what it contends is vague and overly general language in the legislation passed in October with a 22 April effective date.

“Consumers are rewriting the rules when it comes to where they are going for information and the type of information they are looking for,” reports Kaizo’s Anita Nahal, speaking to HBW Insight’s Over the Counter podcast about the agency’s latest Communicating Health Messages report. Trust in social media has increased, and personal beliefs and lived experiences are playing a larger role in shaping attitudes to health information, explains Nahal, who is director at the London-based PR agency. However, consumers are also continuing to “tune-out” from health messages and be less critical of sources. To help consumers navigate this new reality, consumer health companies need to adapt, rethink and reset their communication strategies, Nahal advises.

There’s no simple answer to the question of how to make medicines packaging sustainable, says international recycling consultancy and Veolia Group subsidiary Circpack. “Manufacturers need to assess what works best for them, and recyclers will do the same,” Circpack analyst and circular packaging expert Filipe Vieira de Castro tells HBW Insight in this exclusive Q&A. “The best thing we can all do is put effort into finding common ground between systems, which comes down to better understanding the complexities between them.”
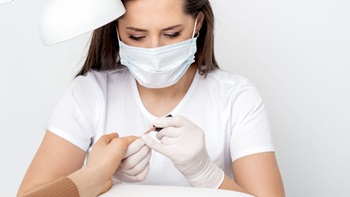
Women’s Voices for the Earth continues to call attention to cosmetic ingredients’ potential health impacts on users including salon workers, while pushing the Cosmetic Ingredient Review to consider a wider variety of safety information in its assessment work.
With NIVEA driving growth in its Consumer division, German firm Beiersdorf AG has signed a multi-year deal with Rubedo Life Sciences to develop anti-aging solutions and new products for the global market. More beauty business news, including L’Oreal, Inter Parfums, Galderma, and LVMH/Sephora sales updates.
The combination of Clasado's Bimuno GOS prebiotic and Probi's Defendum probiotic has been shown to significantly increase lactate levels and increase short chain fatty acid butyrate by 26% - “two important gut health promoting components that are also associated with supporting immune health,” Clasado said.
Sanofi CHC sales up 9% in Q1 driven by addition of US healthy aging supplement brand Qunol. Separately, the Paris-based firm has reached an agreement in principle to resolve the vast majority of Zantac personal injury state court cases in the US.
Walmart Inc. sent a brief to all its beauty and personal-care vendors detailing expectations for brands and other partners under the Modernization of Cosmetics Regulation Act, noting violations of the law breach its supplier agreements.
Seed Health microbiome firm adds medical chief; Veteran CPG executive leads Atlas Bar; Recover Sports connects with QB Murray; Electrolit green flags F1 driver O’Ward; Herbalife scores with Galaxy’s Puig; global sales lead for BioPhotas; and Nutritional product business investment firm adds executive staff.
At-home fertility-related hormone test developer Proov sells equity stake to entertainers and expectant parents Ashanti and Nelly; sexual and vaginal health product firm Good Clean Love moves from its founder as leader to a consumer health industry executive.
R&D investments behind consumer health brands such as Nurofen and Durex pay off for Reckitt in Q1 as it sees volume growth across majority of portfolio.
The Personal Care Products Council seeks to stem the rising tide of titanium dioxide Proposition 65 lawsuits, requesting that a California court prohibit the state’s Attorney General and private enforcers from filing and/or prosecuting new suits against cosmetics companies failing to warn about potential TiO2 exposure.
Firm hosts groundbreaking for 290,000 square-foot global headquarters it’s having built in Summit, NJ, starting with 100,000 square-foot science and innovation and expected to open in 2025. It announced adding Russell Dyer as chief corporate affairs officer starting 13 March.
CRN’s request for clarification, as it continues litigating complaint in US District Court for Southern New York, highlights what it contends is vague and overly general language in the legislation passed in October with a 22 April effective date.
Italy's Recordati saw its OTC sales grow by 10% in 2023 driven by the recovery of the cough & cold market and a growing demand for probiotics.
Firm operating in London, India and United Arab Emirates says its “Ultra Factory” will open in Indiana within the next six months with end-to-end production based on its operational facility in India.
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.