Rx-to-OTC Switch
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Maxwellia Delivers Two UK Menstrual Health Rx-To-OTC Switches
UK switch specialist Maxwellia delivers two “me too” reclassifications in the area of women's health: Evana Heavy Period Relief and Ultravana Period Pain Relief.

Largest PBMs Eliminate Consumers’ Cost Sharing For Opill On Most Commercial Plans
Decisions by “big three” PBMs suggest they’re taking proactive steps to comply with potential HRSA rulemaking to require most health plans, under Affordable Care Act regulations, to cover Opill and other types of OTC birth control without cost sharing.

Poland's President Blocks Rx-To-OTC Switch Of Emergency Contraceptive Pill
Citing a will to protect children's health, Poland's president has blocked a bill which would have restored OTC access to the morning after pill to those aged 15 and above. In response, the health ministry has drawn up a proposal to allow pharmacists to issue prescriptions for emergency contraception.

Perrigo Partnership Makes Opill Star Player On WNBA's Reproductive Health Advocacy Team
Less than a month into launching sales of first oral contraceptive approved for US OTC sales, Perrigo announces multi-year partnership with WNBA focused on building confidence, strength and health equity for women.

FDA Idea To ‘Encourage Competition For New Drugs’ Spares Cutting OTC Switch Market Exclusivity
“FDA is seeking to encourage competition for new drugs by proposing to amend the Hatch-Waxman 3-year exclusivity provisions to ensure that this exclusivity is limited to situations where the new drug applicant is actually seeking such exclusivity,” according to legislative proposals document.
ENVI: Prescription Requirement Only With Evidence Of Antimicrobial Resistance
The EU Parliament's Environment, Public Health and Food Safety committee proposes a “compromise amendment” to the new pharmaceutical directive that would include only antimicrobials for which there is an “identified risk of antimicrobial resistance” to the prescription requirement.
Why Did Germany’s Switch Committee Reject Perrigo’s OTC Daily Contraceptive Proposal?
Skepticism about the need for widening access to the progestogen-only contraceptive pill, fears of associated thrombosis risk with use and the potential cost barriers were among the many reasons for the unanimous rejection of Perrigo's Rx-to-OTC switch application for desogestrel (75μg) for oral use.

Sweden Seeks To Tackle Opioid Overdose Deaths With Naloxone Rx-To-OTC Switch
Opioid antagonist naloxone will soon be available without prescription in Sweden following a successful Rx-to-OTC application from Norway's DNE Pharma.
Poland's Government Pushing To Restore OTC Access To Morning After Pill
As Poland is ranked the worst country in Europe for contraception access by a group of EU parliamentarians, the country's government has voted through a bill which would make the morning after pill available without a prescription for the first time in more than six years.
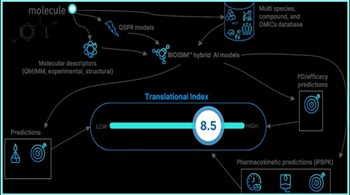
When Using Artificial Intelligence In Pharma R&D, Start With Identifying Problem To Solve
VeriSIM uses generative AI for questions such as changing a drug molecule’s chemistry and machine learning to better predict potential biological implications. “When a child is learning a language, you're teaching a child starting from A-to-Z structures, and then suddenly they start speaking. Then they start learning different things, and then they get into problem solving. That's really what AI is getting really good at now,” says CEO Jo Varshney.

VeriSIM Expects AI Use To Trim Margin For Failure In OTC Switches, Help Boost Revenue Margins
“It's a very expensive process for an OTC drug because you don't make significantly high margins compared to a blockbuster cancer drug. For pharma companies utilizing our platform, you can reduce that efficiency challenge and increase the probability of success,” says VeriSIM CEO Jo Varshney.

Perrigo Ships Opill To Stores, But Contraceptive Access Advocates Say It Doesn’t Deliver On Price
First US OTC daily oral contraceptive shipped to major retailers and pharmacies and will be available on shelves nationwide and online later in March. Perrigo says pre-orders for Opill are available currently from select online retailers.

OTC Naloxone Pioneer Emergent BioSolutions Puts Papa At Helm As It Navigates Strategy Shift
Like his previous move, Papa joins a pharma firm making a significant organizational shift to cut costs and increase revenues after extended period of higher spending and slowing income.
Petros Pharma Introduces AI As ACNU Option In Research For OTC Switch Of Stendra ED Drug
Petros is first to introduce an OTC switch labeling tool which could be the next obvious step hiding in plain sight – AI, which wasn’t noted in FDA’s ACNU proposal and hasn’t previously been linked to a switch.
UK Doctors Call For Mass Market Access To Emergency Contraceptive Pill
UK doctors are calling for the reclassification of oral emergency hormonal contraceptives (EHCs) from pharmacy (P) medicine status to general sales list (GSL) status in the country. Manufacturer Maxwellia, which markets levonorgestrel-based emergency contraception, LoviOne, says it "has what it takes to get this over the line" with the UK Medicines and Healthcare products Regulatory Agency.
Sanofi Awaiting Key Meeting With FDA To Recommence Cialis Switch
Sanofi is waiting to hear from FDA whether it has done enough to recommence its Cialis actual-use trial, which has been on hold for almost two years. The France-based firm gave an update on the Cialis switch program as it reported higher Consumer Healthcare sales in Q4.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.